Photoluminescence Properties of Eu(III) Complexes with Two Different Phosphine Oxide Structures and Their Potential uses in Micro-LEDs, Security, and Sensing Devices: A Review
Volume 8, Issue 3, Page No 154-160, 2023
Author’s Name: Hiroki Iwanagaa)
View Affiliations
Photoluminescence Material Project, New Business Development Office, Next Business Development Div. Toshiba Corporation, 72-34 Horikawa-cho, Saiwai-ku, Kawasaki 212-8585, Japan
a)whom correspondence should be addressed. E-mail: hiroki.iwanaga@toshiba.co.jp
Adv. Sci. Technol. Eng. Syst. J. 8(3), 154-160 (2023); ![]() DOI: 10.25046/aj080317
DOI: 10.25046/aj080317
Keywords: Eu(III) complex, Phosphine Oxide, Red Phosphor, micro-LED, Security, Sensing, Display
Export Citations
In the field of micro-LED displays, there is strong demand for red phosphors with high photoluminescence intensity, high color purity, and small particle size. Here, we focus on Eu(III) complexes because they produce sharp photoluminescence spectra with high color purity and can be dissolved in polymer, enabling a reduction in particle size to the molecular level. We have previously established novel molecular design concepts for Eu(III) complexes by coordinating two different phosphine oxide structures to one Eu(III) ion in order to enhance photoluminescence intensity and increase solubility in polymers and solvents. Many Eu(III) complexes have been developed based on these concepts and their photoluminescence properties investigated. Eu(III) complexes with two different phosphine oxide structures are important candidates for red phosphors in micro-LEDs.
Received: 28 February 2023, Accepted: 20 May 2023, Published Online: 12 June 2023
1. Introduction
Displays require phosphors with high photoluminescence intensity and high color purity. In addition, in micro-LED displays containing ultraviolet (UV) or blue LED arrays and phosphors, where chips are very small, the particle size of phosphors must be sufficiently small to suppress variation in hues among pixels. Therefore, there is a strong demand for a red phosphor that satisfy these conditions. To this end, novel Eu(III) complexes were introduced in a paper originally presented at the 2022 International Conference on Electronics Packaging as a candidate red phosphor for micro-LEDs [1].
In the case of inorganic phosphors, quantum yields decrease with decreasing phosphor particle size because they are present as fine particles in a polymer (Figure 1). Comparison of properties of the Lanthanide complexes and inorganic phosphors are shown in Table 1. The color purity of inorganic phosphors is low because of the large half widths of emission spectra.
Recently, lanthanide complexes, especially Eu(III) complexes, have attracted increasing attention for their application in emission devices, secure media, sensors, and so on [2–8]. Eu(III) complexes are attractive for display use because they produce sharp photoluminescence spectra with high color purity and can reproduce colors in large-area displays.
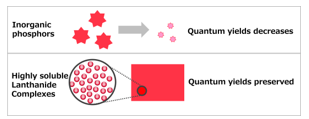
Figure 1: Comparison of inorganic phosphors and highly soluble lanthanide complexes in a polymer.
Table 1: Comparison of properties of the Lanthanide complexes and inorganic phosphors
| Phosphors with small particle size | Quantum yield | Color purity |
| Lanthanide complex | Large | High |
| Inorganic phosphor | Small | Low |
In contrast to inorganic phosphors, particle size is not relevant to the theoretical quantum yield because each molecule of a Eu(III) complex has the function of absorbing and emitting light. From this point of view, Eu(III) complexes are promising candidate red phosphors for micro-LEDs. However, the photoluminescence intensity and solubility of Eu(III) complexes developed to date are insufficient for display use.
An Eu(III) ion itself has very low light absorption and weak emission. However, the emission of lanthanide ions can be enhanced through the antenna effect of ligands. b-diketonates are known to be effective ligands for enlarging photoluminescence intensity of Eu(III) complexes. b-Diketonates absorb light and transfer energy to lanthanide ions efficiently [9, 10]. The photoluminescence intensity of Eu(III) complexes depends largely on the substituents on the b-diketonates because the triplet-state energy levels of b-diketonates are derived from the molecular structures of the substituents. However, it can be difficult to obtain sufficient emission intensity for use in emission devices simply by adjusting the substituents of b-diketonates.
There are two main types in ligands of lanthanide complexes. One is ionic ligands and the other is non-ionic ligands. b-diketonates are prominent ionic ligands and neutralize the charge of lanthanide ions. It is known that photoluminescence intensities are enhanced by the effects of non-ionic ligands in addition to b-diketonates. Phosphine oxide compounds are strong Lewis bases and excellent non-ionic ligands for enlarging photoluminescence intensity [11] (Figure 2).
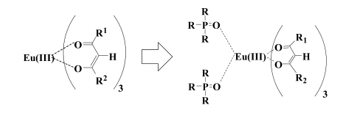
Figure 2: Coordinating phosphine oxides to a Eu(III) ion.
However, there is room for further improvements in emission intensity. At the same time, the solubility of Eu(III) complexes with two identical phosphine oxides are too low to be dissolved in polymers or solvents. For this reason, Eu(III) complexes with low solubility have limited applications.
2. Experimental Section
2.1 Measurement of photoluminescence and excitation spectra
Photoluminescence and excitation spectra were measured at room temperature using a spectrofluorometer (Fluoromax 4, Horiba Jobin Yvon Inc.). Excitation and emission slit widths were set to 0.5 nm for measurement of emission spectra, and to 0.7 and 0.6 nm for measurement of excitation spectra, respectively. Measurement intervals are 1 nm. Scanning rate are 600 nm/min. Dark offset and corrections were applied to both the emission and excitation sites.
2.2 Measurement of emission lifetimes
Measurement of emission lifetimes were performed as follows. Each solution of the Eu(III) complexes was placed in a sealed cell and measured using the spectrofluorometer with the excitation wavelength set to 370 nm. Single exponential functions were used to fit the relative decay curves monitored at the maximum wavelength in order to calculate the emission lifetimes. c2 values were in the range of >1.0 and <1.2.
2.3 Measurement of absolute quantum yields
Total absolute quantum yields (FTOT) were measured using a photonic multichannel analyzer (PMA-12 C10027-01, Hamamatsu Photonics K.K). An integrating sphere was used for all measurements.
3. Results and Discussion
3-1. Eu(III) complexes with two different phosphine oxides
Figure 3 shows the relationships between molecular structures and photoluminescence spectra of Eu(III) complexes. The photoluminescence intensity of a Eu(III) complex with no phosphine oxide is usually very small, but when two triphenyl phosphine oxides coordinate, it increases to some extent. Furthermore, when two tributyl phosphine oxides coordinate, photoluminescence intensity increases further, and when both triphenyl and tributyl phosphine oxides coordinate, photoluminescence intensity becomes much higher [12]. The important point here is that coordination of two different phosphine oxide ligands is effective for increasing photoluminescence intensity [12–14]. Eu(III) complexes with two different phosphine oxides can be dissolved and are homogeneous at the molecular level in polymers. Polymers containing our Eu(III) complexes are colorless and transparent under room light but emit a pure color when irradiated with UV and 464-nm light.
3.2. Eu(III) complexes with an asymmetric diphosphine dioxide ligand
We detected the ligand exchange of phosphine oxide in Eu(III)-b-diketonates by NMR analysis [13]. However, ligand exchange is expected to have an undesirable effect on durability. To overcome this problem, we developed asymmetric diphosphine dioxide ligands (Figure 4). They have molecular structures consisting of two different phosphine oxide parts and methylene units and suppress ligand exchange via the chelate effect. In addition, the photoluminescence intensity of Eu(III)-b-diketonates with an asymmetric diphosphine dioxide ligand is higher than that with two different phosphine oxides [15, 16].
Tb(III) complexes with two different phosphine oxides or a single asymmetric diphosphine dioxide were also investigated [17]. It was found that solubilities of Tb(III) complexes were increased by coordination of two different phosphine oxide structures. However, photoluminescence intensities are strongly dependent on the substituents of b-diketonates because of the strong influences of back-energy transfer from excited Tb(III) ions to the ligands.
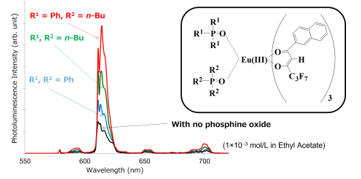
Figure 3. Comparison of the photoluminescence spectra of Eu(III) complexes in ethyl acetate at a concentration of 2×10-4 mol/L at room temperature. Showing the effects of phosphine oxides and their combination on photoluminescence intensity [12].
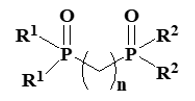
Figure 4. Molecular structures of the asymmetric diphosphine dioxide ligand that increase the photoluminescence intensity of Eu(III) complexes (R1=aromatic substituent, R2=aliphatic substituent).
3-3. Solubility of Eu(III) complexes with phosphine oxide ligands
Relationships between the molecular structures of Eu(III) complexes and solubility in solvents were investigated [18]. Eu(III) complexes with two different phosphine oxides are highly soluble in solvents and can be dissolved even in a fluorinated solvent. However, the solubility of Eu(III) complexes with an asymmetric diphosphine dioxide ligand is lower than that of Eu(III) complexes with two different phosphine oxides. We found that meta-substitution of trifluoromethyl groups (CF3) on the phenyl groups of diphosphine dioxide ligands produces outstanding effects in terms of enhancing the solubility of Eu(III) complexes [19]. Similarly, the solubility of anthraquinone dichroic dyes in fluorinated media are markedly enhanced by the substitution of CF3 groups [20–22].
3.4. Photoluminescence properties of Eu(III) complexes with an asymmetric diphosphine dioxide ligand
Figure 5 shows the optimal diphosphine dioxide ligand for Eu(III) complexes that increases both quantum yields and solubility [23]. Having CF3 groups at the meta position of phenyl groups is one of the most important characteristics for achieving both high quantum yield and high solubility. Figures 6 and 7 show the relationships between excitation wavelength and quantum yields of Eu(III) complexes with and without diphosphine dioxide ligands, respectively [23].
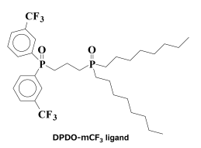
Figure 5. Molecular structure of the asymmetric diphosphine dioxide ligand for Eu(III) complexes that increase the photoluminescence intensity (DPDO-mCF3 ligand) [23].
The maximum total photoluminescence quantum yield (FTOT) of Eu(III)(hfnh)3 is small and the solid-state FTOT of Eu(III)(hfnh)3 is smaller than the solution-state FTOT caused by concentration quenching (Figure 6). In contrast, FTOT of Eu(III)(hfnh)3(DPDO-mCF3) is much greater than that of Eu(III)(hfnh)3. Furthermore, FTOT is greater in the solid state than in the solution state. By coordinating the diphosphine dioxide ligands, quantum yields increase eminently. In the solid state, the maximum quantum yield reaches 0.82.
Diphosphine dioxide ligand functions as a separator, maintaining the distance among Eu(III) ions that prevent concentration quenching. In the solid state, there are no solvent molecules to decrease FTOT of the Eu(III) complexes.
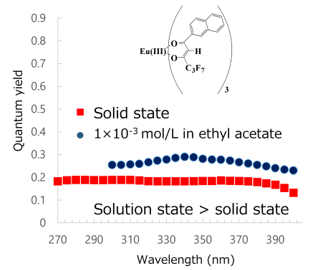
Figure 6. Action spectra (excitation wavelength vs. FTOT) of Eu(III)(hfnh)3 in the solid and solution states [23].
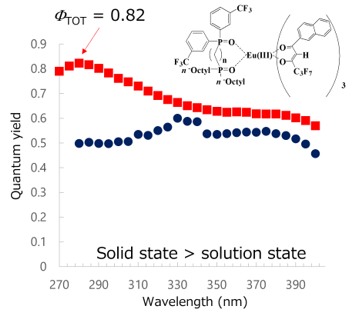
Figure 7. Action spectra (excitation wavelength vs. FTOT) of Eu(III)(hfnh)3(DPDO-mCF3) in the solid and solution states [23].
3.5. Quantum yields of Eu(III) complexes with thienyl substituted diphosphine dioxide
Thienyl groups are electron-donating substituents that are expected to enhance the Lewis basicity of the oxygen atoms in diphosphine dioxide ligands. Dithienyl[3-(dioctylphosphinyl)-propyl] phosphine oxide (DTDOPO) and dithienyl[5-(dibutyl-phosphinyl)pentyl]phosphine oxide (DTDBPO) ligands were developed with the aim of forming stronger coordinate bonds with the Lewis acid Eu(III). A diphenyl[3-(dioctylphosphinyl)propyl]phosphine oxide (DPDO) ligand with phenyl groups instead of thienyl groups was prepared for comparison (Figure 8) [24].
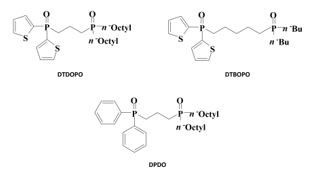
Figure 8. Molecular structures of thienyl-substituted and phenyl-substituted diphosphine dioxides [24].
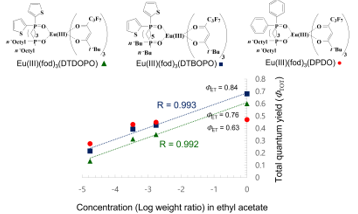
Figure 9. Quantum yields of Eu(III) complexes with thienyl-substituted and phenyl-substituted diphosphine dioxides both in the solid state and in solution (ethyl acetate) [24].
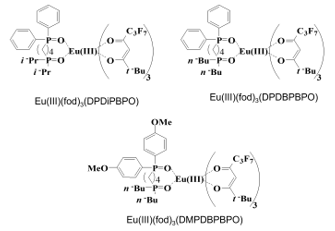
Figure 10. Molecular structures of Eu(III) complexes with a diphosphine dioxide ligand [25]. DPDiPBPO: diphenyl[4-(diisopropylphosphinyl)butyl]phosphine oxide, DPDBPBPO: diphenyl[4-(dibutylphosphinyl)butyl]phosphine oxide, DMPDBPBPO: di(4-methoxyphenyl)[4-(dibutylphosphinyl)butyl]phosphine oxide.
Figure 9 shows the relationships between concentrations in ethyl acetate and quantum yields of Eu(III)(fod)3(DTDOPO), Eu(III)(fod)3(DTBOPO), and Eu(III)(fod)3(DPDO). The concentrations and quantum yields have a strong positive linear correlation and the quantum yields in the solid state (point with concentration [Log weight ratio] 0) are located on the extended line for Eu(III)(fod)3(DTDOPO) and Eu(III)(fod)3(DTBOPO) with thienyl groups. No concentration quenching was observed. As the concentration of Eu(III)(fod)3(DPDO) increases, the differential coefficients become smaller. Research investigating the special feature of Eu(III) complexes with thienyl groups in the solid state is ongoing.
3.6. Effects of alkyl groups in diphosphine dioxide ligands on the photoluminescence properties of Eu(III) complexes
To investigate the effects of the molecular structures of diphosphine dioxide ligands on the photoluminescence properties of Eu(III) complexes, we prepared three Eu(III) complexes with the same molecular structure except for the slight difference in diphosphine dioxide ligands shown in Figure 10 [25].
Eu(III)(fod)3(DPDiPPBPO) has i-propyl groups in a diphosphine dioxide ligand, while Eu(III)(fod)3(DPDBPBPO) and Eu(III)(fod)3(DMPDBPBPO) have n-butyl groups.
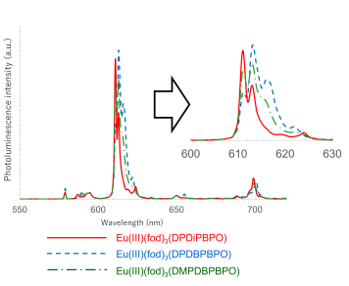
Figure 11. Photoluminescence spectra of the Eu(III) complexes Eu(III)(fod)3(DPDiPBPO), Eu(III)(fod)3(DPDBPBPO), and Eu(III)(fod)3(DMPDBPBPO) in the solid state. They are excited at 370 nm [25].
The photoluminescence spectra of the Eu(III) complexes Eu(III)(fod)3(DPDiPBPO), Eu(III)(fod)3(DPDBPBPO), and Eu(III)(fod)3(DMPDBPBPO) in the solid state are shown in Figure 11. The shapes of the Stalk splitting of the 5D0→7F2 transition differ among them. Of note, the half-width of the 5D0→7F2 transition of Eu(III)(fod)3(DPDiPBPO) with i-propyl (i-Pr) substituted for the diphosphine dioxide ligand was 2 nm and conspicuously smaller compared with Eu(III)(fod)3(DPDBPBPO) and Eu(III)(fod)3(DMPDBPBPO) with n-butyl substituted for the diphosphine dioxide ligand. The smaller half-width means the ligand field has a higher symmetry.
Table 2. Photoluminescence properties of the Eu(III) complexes [25]
| Solid state | |||
| Ligand | DPDiPBPO | DPDBPBPO | DMPDBPBPO |
| texp (ms)a | 0.99 | 0.84 | 0.89 |
| kexp (s–1)b | 1009 | 1193 | 1129 |
| trad (ms)c | 1.37 | 0.99 | 1.09 |
| krad (s–1)d | 729 | 1012 | 992 |
| knrad (s–1)e | 280 | 181 | 207 |
| FLnf | 0.72 | 0.85 | 0.82 |
| FETg | 0.81 | 0.83 | 0.76 |
| IMD/ITOTh | 0.0678 | 0.0488 | 0.0537 |
| FTOTi | 0.58
(345 nm) |
0.70
(350 nm) |
0.62
(345 nm) |
| Ratio Rj | 11.1 | 16.7 | 14.9 |
aExperimental lifetime measured in solid. c2 values were in the range of > 1.0 and < 1.2.
bexperimental decay rate
cRadiative lifetime calculated using the formula
trad = 1/n3AMD,0 ´ IMD/ITOT (n= 1.50).
dRadiative decay rate
eNon-radiative decay rate
fIntrinsic quantum yield calculated using the formula
FLn = texp / trad.
gEnergy transfer efficiency
hRatio between the integrated intensity of the 5D0→ 7F1 transition (IMD) and the total integrated emission intensity 5D0→ 7FJ(J = 0-6) (ITOT)
iTotal quantum yield measured in solid state. (Peak wavelength of the action spectrum (wavelength vs. quantum yield)).
jCalculated from the formula I(5D0→ 7F2) / I(5D0→ 7F1)
Table 2 shows the photoluminescence properties of the Eu(III) complexes. The FTOT of Eu(III)(fod)3(DPDiPBPO) was smaller than that of others because of the smaller intrinsic quantum yield (FLn). The smaller ratio R and larger IMD/ITOT of Eu(III)(fod)3(DPDiPBPO) showed that the Eu(III) complex with i-Pr groups in the diphosphine dioxide ligand had a higher symmetry in ligand fields compared with the others in the solid state. These results agree well with the result of the smaller half-width of Eu(III)(fod)3(DPDiPBPO). These noticeable differences in properties are caused by the difference in molecular structures between the i-Pr and n-Bu groups in diphosphine dioxides.
Based on the above, we propose a hypothesis about FTOT and diphosphine dioxide ligand structures: the steric hindrance of diphosphine dioxide ligands with n-Bu groups is larger than that of ligands with i-Pr groups, and a larger steric hindrance causes diphosphine dioxide ligands to have lower symmetry of the ligand field, thereby inducing a larger FTOT. In the next section, we focus on the effects of steric hindrance in diphosphine dioxide ligands.
3.7. Elucidation of the effects of diphosphine dioxide ligands on the quantum yield and photoluminescence intensity of a 6-coordinate Eu(III)-b-diketonate complex
To elucidate the coordination effects of phosphine oxide ligands, the following 6-coordinate Eu(III) complex designed to have low luminescence and a large absorption coefficient was synthesized: (Tris{6,6,7,7,8,8,8-heptafluoro-1-[2-(9,9-dimethylfluorenyl)]-1,3-octanedionate} europium(III) (Eu(III)(hfod)3) (Figure 12) [26].
Dimethylfluorenyl groups are bulky aromatic substituents with large absorption coefficients. Partially fluorinated alkyl groups in b-diketonates are also very bulky. The methylene units in partially fluorinated alkyl groups have the function of decreasing the energy transfer efficiency from the ligands to the Eu(III) ion.
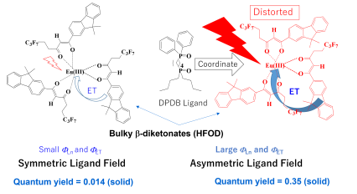
Figure 12. Coordinating effects of DPDB ligand with Eu(III)(hfod)3 with bulky b-diketonates, “hfod” [26].
The photoluminescence intensity of Eu(III)(hfod)3 is dramatically enhanced by coordinating a DPDB ligand (generating Eu(III)(hfod)3(DPDB)) due to the increased FTOT, FLn, and FET in both the solution and solid states.
We propose the following hypothesis for the increase in FTOT of 6-coordinate Eu(III) complex caused by the effects of the DPDB ligand. When a DPDB ligand coordinates with the Eu(III) ion, the positions of the nearest oxygen atoms around the Eu(III) ion are shifted by steric repulsion, and the relative positions of the nearest oxygen atoms become distorted. Ligand field is asymmetrized by the distorted coordination environment, and that increases FTOT.
The norm of the effective dipole moment of the ligand field μ was defined [26]. We demonstrated that the energy transfer efficiencies from the lowest triplet state of the ligands to the 5D1 level of the Eu(III) ion (ΦET) increases when the ligand fields of the Eu(III) ion become more asymmetric by coordinating the DPDB ligand.
3.8. High-sensitivity method for detecting the pesticide dichlorvos by using Eu(III)-b-diketonate as a quenching probe
Dichlorvos is a general-purpose insecticide with agricultural, household, and animal applications. However, it is harmful to humans, and thus a high-sensitivity method for detecting dichlorvos that provides results in a short time would be desirable.
We found that Eu(III)(hfnh)3 was a highly sensitive luminescent probe for the pesticide dichlorvos. The photoluminescence intensity of Eu(III)(hfnh)3 was drastically and rapidly decreased when a dilute solution of dichlorvos was added to the solutions of Eu(III)(hfnh)3 (Figure 13) [27].
When a solution of dichlorvos was mixed with a solution of Eu(III)(hfnh)3 and shaken, FET drastically decreased (0.64→ 0.15). The photoluminescence quenching of Eu(III)(hfnh)3 by dichlorvos occurred before the energy transfer from b-diketonates to a Eu(III) ion between the dichlorvos molecules and the b-diketonates
The photoluminescence of Eu(III)(hfnh)3 is not quenched by compounds with similar structures and has a favorable selectivity for dichlorvos. These results indicate that Eu(III)(hfnh)3 is a strong candidate for a sensitive, selective, and quick method for detecting dichlorvos. Other organophosphorus pesticides can be selectively detected by other Eu(III)-b-diketonates.
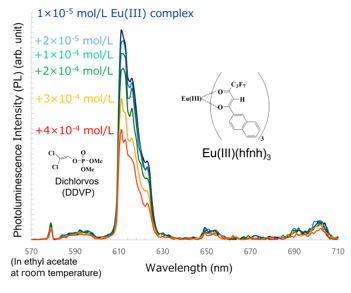
Figure 13. Photoluminescence quenching of Eu(III)(hfod)3 by the pesticide dichlorvos [27].
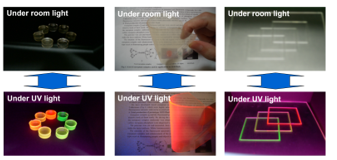
Figure 14. Colorless and transparent photoluminescence materials containing our Eu(III) complexes and/or Tb(III) complexes in a polymer [16].
3.9. Characteristics of colorless and transparent photoluminescence materials involved in our Eu(III) complexes and/or Tb(III) complexes in a polymer
We developed multiple lanthanide complexes having two different phosphine oxides or an asymmetric diphosphine dioxide that improved both photoluminescence intensity and solubility. When Eu(III) or Tb(III) complexes or both are dissolved in a polymer, materials that are colorless and have transparent photoluminescence under room light are produced. These materials emit pure red or green as well as yellow and orange intermediate colors when irradiated with UV or near-UV light (Figure 14) [16].
LED devices comprising a UV-light LED chip and a fluorescent layer consisting of a fluorinated polymer and Eu(III) complexes with two different phosphine oxides were prototyped. The developed devices emit a pure red color. The highest luminous flux obtained under optimum conditions, which to our knowledge is the best result reported as of 2007, was 870 m lumen/20 mA, when excited by a 402-nm LED chip (Figure 15) [28].
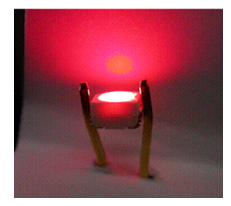
Figure 15. LED devices containing novel Eu(III) complexes in the fluorinated layer [28].
4. Conclusion
We found that coordination of two different phosphine oxide structures to a lanthanide ion is effective for enhancing the photoluminescence intensity and solubility of lanthanide complexes. An asymmetric diphosphine dioxide ligand consisting of two different phosphine oxide parts and methylene units produce further excellent effects in terms of enhancing the quantum yields and photoluminescence intensity of Eu(III) complexes. Asymmetric diphosphine dioxide ligands induce asymmetry in the ligand fields of Eu(III) complexes, thereby improving the quantum yields. Colorless and transparent photoluminescence materials can be obtained by dissolving Eu(III) complexes in polymers or solvents. These materials show great promise for use in LEDs as well as security and sensing devices.
We believe that our Eu(III) complexes have advantages over inorganic phosphors as red phosphors for use in micro-LED displays.
Acknowledgment
The author would like to thank Okuno Atsushi, Takahisa Kobayashi, Junichi Washizuka, Chiari Shimizu, Naruaki Watanabe, Akiko Yuzawa, and Huang Chingchun for fruitful discussions.
- H. Iwanaga, “Photoluminescence Properties of Eu(III) Complexes with an Asymmetric Diphosphine Dioxide Ligand for Potential Uses in LED, Security, and Sensing Devices,” 2022 International Conference on Electronics Packaging (ICEP), 17-18, 2022, doi:10.23919/ICEP55381.2022.9795463.
- Q. Xin, W.L. Li, G.B. Che, W.M. Su, X.Y. Sun, B. Chu, B. Li, “Improved Electroluminescent Performances of Europium-Complex Based Devices by Doping into Electron-Transporting/Hole-Blocking Host,” Applied Physics Letters, 89, 223524, 2006, doi:10.1063/1.2400112.
- Q. Xin, W.L. Lia, W.M. Su, T.L. Li, Z.S. Su, B. Chu, B. Li, “Emission Mechanism in Organic Light-Emitting Devices Comprising a Europium Complex as Emitter and an Electron Transporting Material as Host,” Journal of Applied Physics, 101, 044512, 2007, doi:10.1063/1.2655225.
- G. Santos, L.G. Paterno, F.J. Fonseca, A.M. Andrade, L.F. Preira, “Enhancement of Light Emission from an Europium(III) Complex Based-OLED by Using Layer-by-Layer Assembled Hole-Transport Films,” ECS Transactions, 39, 307-313, 2011, doi:10.1149/1.3615207.
- S.G. Liu, W.Y. Su, R.K. Pan, X.P. Zhou, “Red Emission of Eu(III) Complex Based on 1-(7-(tert-butyl)-9-ethyl-9H-carbazol-2-yl)-4,4,4-trifluorobutane1,3-dione Excited by Blue Light,” Chinese Journal of Chemical Physics, 25, 697-702, 2012, doi:10.1088/1674-0068/25/06/697-702.
- A.M. Kaczmarek, Y.Y. Liu, C. Wang, B. Laforce, L. Vincze, P.V.D. Voort, K.V. Hecke, R.V. Deun, “Lanthanide “Chameleon” Multistage Anti-Counterfeit Materials,” Advanced Functional Materials, 27, 1700258, 2017, doi:10.1002/adfm.201700258.
- X. Li, J. Gu, Z. Zhou, L. Ma, Y. Tang, J. Gao, Q. Wang, “New Lanthanide Ternary Complex System in Electrospun Nanofibers: Assembly, Physico-Chemical Property and Sensor Application,” Chemical Enginerring Journal, 358(15), 67-73, 2019, doi:10.1016/j.cej.2018.10.003.
- G. Lu, X. Kong, C.M. Wang, L.Y. Zhao, D.D. Qi, Y.Y. Jiang, S. Zhao, Y.L. Chen, J.Z. Jiang, “Optimizing the Gas Sensing Properties of Sandwich-Type Phthalocyaninato Europium Complex Through Extending the Conjugated Framework,” Dyes and Pigments, 161, 240-246, 2019, doi:10.1016/j.dyepig.2018.09.062.
- S. Sato, M. Wada, T. Seki, “Some properties of europium β-diketone chelates 1. (Synthesis and fluorescent properties),” Japanese Journal of Applied Physics, 7, 7-13, 1968, doi: 10.1143/JJAP.7.7
- S. Sato, M. Wada, “Relation between intramolecular energy transfer efficiencies and triplet state energies in rare earth β-diketone chelate,” Bulletin of the Chemical Society of Japan, 43, 1955-1962, 1970, doi:10.1246/bcsj.43.1955.
- J. Yuan, K. Matsumoto, “Fluorescence Enhancement by Electron-Withdrawing Groups on β-Diketones in Eu(III)-β-diketonato-topo Ternary Complexes”, Analytical Sciences, 12, 31–36, 1996, doi:10.2116/analsci.12.31.
- H. Iwanaga, “Investigation of strong photoluminescence and highly soluble Eu(III) complexes with phosphine oxides and β-diketonates,” Journal of Luminescence, 200, 233-239, 2018, doi:10.1016/j.jlumin.2018.03.070.
- H. Iwanaga, A. Amano, M. Oguchi, “Study of molecular structures and properties of europium(III) complexes with phosphine oxides by NMR analysis,” Japanese Journal of Applied Physics, 44, 3702-3705, 2005, doi:10.1143/JJAP.44.3702.
- H. Iwanaga, A. Amano, F. Aiga, K. Harada, M. Oguchi, “Development of Ultraviolet LED Devices Containing Europium (III) Complexes in Fluorinated Layer,” Journal of Alloys and Compounds, 408–412, 921-925, 2006, doi:10.1016/j.jallcom.2005.01.138.
- H. Iwanaga, F. Aiga and A. Amano, “The molecular structures and properties of novel Eu(III) complexes with asymmetric bis-phosphine oxides,” Materials Research Society symposia proceedings, 965, 211-216, 2006, doi:10.1557/PROC-0965-S03-12.
- H. Iwanaga, “Emission properties, solubility, thermodynamic analysis and NMR studies of rare-earth complexes with two different phosphine oxides,” Materials, 3, 4080-4108, 2010, doi:10.3390/ma3084080.
- H. Iwanaga, and F. Aiga, “Novel Tb(III) complexes with two different structures of phosphine oxides and their properties”, Journal of Luminescence, 130(5), 812-816, 2010, doi:10.1016/j.jlumin.2009.11.039.
- H. Iwanaga, A. Amano, F. Furuya, and Y. Yamasaki, “Solubility in fluorinated medium and thermal properties of Europium(III) complexes with phosphine oxides,” Japanese Journal of Applied Physics, 45, 558-562, 2006, doi:10.1143/JJAP.45.558.
- H. Iwanaga, “Relationships between molecular structures of aromatic- and aliphatic-substituted diphosphine dioxide ligands and properties of Eu(III) complexes,” Optical Materials, 85, 418-424, 2018, doi:10.1016/j.optmat.2018.08.071.
- H. Iwanaga, K. Naito, and Y. Nakai, “The Molecular structures and properties of anthraquinone-type dichroic dyes,” Molecular Crystals and Liquid Crystals, 364, 211-218, 2001, doi:10.1080/10587250108024989.
- H. Iwanaga, K. Naito, and F. Aiga, “Properties of novel yellow anthraquinone dichroic dyes with naphthylthio groups for guest–host liquid crystal displays,” Journal of Molecular Structure, 975, 110-114, 2010, doi:10.1016/j.molstruc.2010.04.003.
- H. Iwanaga, and F. Aiga, “Correlations among molecular structures, solubilities in fluorinated media, thermal properties and absorption spectra of anthraquinone dichroic dyes with phenylthio and/or anilino groups,” Liquid Crystals, 38(2), 135-148, 2011, doi:10.1080/02678292.2010.531149.
- H. Iwanaga, “A CF3-substituted Diphosphine Dioxide Ligand that Enhances both Photoluminescence Intensity and Solubility of Eu(III) Complexes,” Journal of Alloys and Compounds, 790, 296-304, 2019, doi:10.1016/j.jallcom.2019.03.085.
- H. Iwanaga, “Photoluminescence Properties of Eu(III) Complexes with Thienyl-Substituted Diphosphine Dioxide Ligands,” Bulletin of the Chemical Society of Japan, 92(8), 1385-1393, 2019, doi:10.1246/bcsj.20190068.
- H. Iwanaga, “Effects of Alkyl Groups in Diphosphine Dioxide-Ligand for Eu(III)-β-Diketonate Complexes on Photoluminescence Properties,” Chemical Physics Letters, 736, 136794, 2019, doi:10.1016/j.cplett.2019.136794.
- H. Iwanaga and F. Aiga, “Quantum Yield and Photoluminescence Intensity Enhancement Effects of Diphosphine Dioxide Ligand on a 6-Coordinate Eu(III)-β-Diketonate Complex with Low Luminescence,” ACS Omega, 6(1), 416–424, 2021, doi:10.1021/acsomega.0c04826.
- H. Iwanaga and F. Aiga, “A Simple and Sensitive Detection Method for the Pesticide Dichlorvos in Solution using Eu(III)-β-Diketonate as a Luminescent Probe,” Japanese Journal of Applied Physics, 59, SDDF05, 2020, doi:10.7567/1347-4065/ab5c96.
- H. Iwanaga, and A. Amano, “Solid-State 31P-Nuclear Magnetic Resonance Analysis of Eu(III) Complexes with Phosphine Oxides in Fluorinated Polymer,” Japanese Journal of Applied Physics, 46, L495-L497, 2007, doi:10.1143/JJAP.46.L495.
Citations by Dimensions
Citations by PlumX
Google Scholar
Crossref Citations
- Georgia G. Sands, Alyssa K. Cook, Angelina Delabbio, Tim Fuhrer, Matthew D. Bailey, Erin G. Leach, Isabella R. Purosky, Shannon M. Biros, "Three derivatives of phenacyldiphenylphosphine oxide: influence of aromatic and alkyl substituents on the luminescence sensitization of four Ln(NO3)3 salts." Dalton Transactions, vol. 53, no. 7, pp. 3118, 2024.
No. of Downloads Per Month
No. of Downloads Per Country
