Waste To Energy Feedstock Sources for the Production of Biodiesel as Fuel Energy in Diesel Engine – A Review
Volume 6, Issue 1, Page No 409-446, 2021
Author’s Name: Maroa Semakulaa), Freddie Inambao
View Affiliations
University of KwaZulu-Natal, Mechanical Engineering department, Durban, 4041, South Africa
a)Author to whom correspondence should be addressed. E-mail: ssemakulamaroa@gmail.com
Adv. Sci. Technol. Eng. Syst. J. 6(1), 409-446 (2021); ![]() DOI: 10.25046/aj060147
DOI: 10.25046/aj060147
Keywords: Biodiesel Families, Sources of Biodiesel, Socio-Economic Opportunities Production and Utilization, Municipal Solid Waste, Waste to Energy
Export Citations
In the recent past, there has been a renewed shift into biomass and other recycled waste sources for biodiesel production and utilization. This is a critical area of research and study in which this present work intends to review and identify gaps in literature by shifting the focus of review to non-plant based sources for biodiesel production. Traditional biodiesel feedstock sources have always presented a conflict of food security versus energy. This shift will be identified in literature to see if change to non-plant based feedstocks sources has increased food security by discouraging the contribution of commercial farming for the production of biodiesel. This work will identify biodiesel families, generations, traditional and non-traditional feedstocks for biodiesel production. It will also discuss the non-edible biodiesel feedstocks sources in relation to waste to energy recovery. The other factor this work will review is to study how the use of non-plant based feedstocks such as municipal solid waste has improved environmental protection by reducing pollution and landfilling. In other words, this work will review the impact of Using waste municipal solid biomass resources such as waste tyres and waste plastics and changing them into energy sources. This review study aims at increasing environmental awareness, sustainability and reporting the progress made in waste to energy policy shift in many countries globally. This review will look at socio-economic opportunities in recycling besides the academic and research impacts of waste to energy policies adopted in many countries. The review will climax with a conclusion and future trends in waste to energy in relation to municipal solid waste resources.
Received: 28 September 2020, Accepted: 04 December 2020, Published Online: 22 January 2021
1. Introduction and Historical Background of Biodiesel
The history of biodiesel fuel has its route traced to the discovery of the diesel engine by the German engineer Rudolf Diesel in the 1890s. However, the first use of vegetable oil as a fuel was in the world exposition in Paris in 1900. This was at the request of the French government, which had an interest in development of fuels of local origin for its African colonies for energy and power generation independence. There were a total of 5 engine models displayed and tested during the exposition [1]. After the Second World War, several literatures report the use of vegetable oil in diesel engines and operational difficulties encountered with their application as combustion fuel [2-5].
These reports led to the award of the Belgium patent 422877 in 1937 to Chavanne. However, after the second world war the alternative fuel research took a lull until the energy crisis of the 1970s when [6] came up with work on esters of vegetable oil. Using sunflower oil in a diesel engine, they reported that use of sunflower methyl esters eliminated the problems of viscosity and operational issues. Although fossil-based fuels have been playing a major role in the growth of industries, transportation and agricultural activities, the future of fossil fuel as primary source of energy is not sustainable due to depletion, outstripped demand of fuel energy consumption and use. Fossil based fuel have more qualities that make them appeal to the user readily such as availability, good combustion properties and high heating values [7]. Energy estimates from the international energy agency puts the estimates of the growth of energy consumption at 53 % by the year 2030 [8, 9].
In the USA the energy information agency (EIA) projects liquid fuel consumption to increase from 86.1 million barrels per day to 110.6 MBD by the year 2035 [10]. The depletion of fossil fuel reserves has occupied world energy forums and decision makers. Therefore, there has been rapid research development in green alternative fuel energy, which is renewable, domestically available, environmentally friendly and feasible technically. Biodiesel therefore has become technically a better choice for researchers as they contain characteristics and identical physical properties to fossil fuel especially diesel fuel. This makes the future of biodiesel more tenable and more promising as sources of alternative fuel energy especially in developing countries. These countries experience a heavy burden on the importation of liquid petroleum fuel for energy, environmental impacts and effects of pollution on the public human health [11]. Table 1 showing a global production of biodiesel in 2015 and the unit cost of production.
Table 1: Worldwide production of biodiesel with cost [12]
| Country | Estimated potential (Litres) | Production Cost($/l) |
| Brazil | 2,567,000,000 | 0.62 |
| Indonesia | 7,595,000,000 | 0.49 |
| Argentina | 5,255,000,000 | 0.62 |
| Malaysia | 14,540,000,000 | 0.53 |
| USA | 3,212,000,000 | 0.85 |
| Netherlands | 2,496,000,000 | 0.75 |
| Germany | 2,024,000,000 | 0.79 |
| Philippines | 1,234,000,000 | 0.53 |
| Belgium | 1,213,000,000 | 0.78 |
| Spain | 1,073,000,000 | 1.71 |
The importance of modern-day transport systems cannot be gain said, especially the transportation of goods and services and people. The propulsion provided by internal combustion engines with diesel fuel as the primary source of energy, forms the bulk of commercial use and now personal transport, owing to their numerous advantages as compared to other forms or types of propulsion by internal combustion engines. Diesel engines are inherently lean burn engines, and emit relatively low carbon dioxide emissions as compared to petrol propelled internal combustion engines. Other advantages offered by diesel engines include high thermal efficiencies, durability and construction robustness [13]. This makes their continued increase and expansion as more countries move into urbanization, industrialization, and catching up with the highly industrialized countries. However, there has been a formidable challenge to phase them out, based on environmental and human health issues due to the high levels of NOX, smoke and PM emissions.
Diesel engines have shown to run stably on most medium blended ratios of waste plastic oil, although they produce more NOX, UHC and CO emissions. However to stabilize their performance for higher blend ratios, injection timing has been proposed as a method of achieving engine performance stability without upgrading of fuel, engine modification or fuel alteration through addition of additives as observed by [14]. Injection timing was seen to affect performance from WPPO Jatropa blends of 20% tyre oil and 80% Jatropa ester oil resulting into lower fuel consumption, CO, UHC and PM although NOX emissions increased [15]. Nevertheless In a research study by [16] the authors report increased BTE and NOX emissions, thus concurring with the findings of Sharma et al. (2015) on emissions of NOx, but decreased results on fuel consumption, CO, and UHC.
The continued increase in stringent emission regulations enacted by global industrial powers, United States of America and the European Union environmental protection agencies including the G-7 and G-20. Have since labelled the diesel engine as primary polluter in the transport sector. Hence the clamour for alternative fuels, which mitigate pollution, are sought in the interest of reducing energy consumption, environmental degradation and air pollution from NOX gases, which diesel engines emit, thus decelerating atmospheric carbon concentration globally. The road transport sector is an environmental concern, since its rapid expansion is fast eroding all the technological developments and improvements thus so far achieved in the war against pollution from diesel-propelled engines.
Across the globe, significant resources have been mobilized to find alternative sources of energy. Different regions and countries have focused attention at sources of supply and technical processes that give them comparative advantages. In sub-Saharan Africa, renewable energy alternatives for transportation have huge potential sources, one of which is biodiesel derived from municipal solid waste sites, waste tires, waste engine used oil and waste cooking oil. Considerable research has been ongoing in this area but some gaps have not been addressed. There is a clear need to conduct evaluations that enable precise technical classification of the performance and emission of biodiesel derived from the waste sources mentioned above. Figure 1 is showing available general waste data (from municipalities) in South Africa from 1997 to 2011.
This will enable us to filter the data, by comparing it with local South African standards and regulations with global standards and regulations. Through this, challenges can be identified and plans of action mapped out to tackle them. It is necessary to identify and assemble the right set of tools and technique needed to conduct studies on these municipal solid waste feedstock sources in a manner that optimizes them as available resources. The stated goal of this work is to extract, information on solid waste municipal sources for the production of biodiesel using thermal processes. This information is contained in many published research articles and will be presented here as a review and future development.
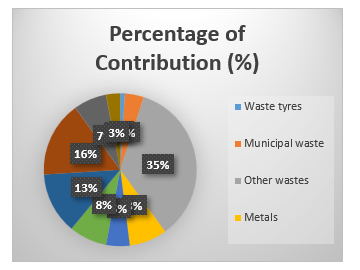 Figure 1: Percentage contribution of each waste stream of general solid Analysed from available data 2017 (from municipalities) in South Africa [17]
Figure 1: Percentage contribution of each waste stream of general solid Analysed from available data 2017 (from municipalities) in South Africa [17]
2. Biodiesel Families and Generations
Traditionally families come in generations defined by the length of time. As such, biofuels have lumped into families or generational categories. Production and use of biofuels has gained significant awareness and attention in academia, industry and government policy makers. Globally 28 countries including developing countries have been prominent in enacting biofuel mandated policies with substantial tax subsidies for biofuels [18]. Biofuels shift targets displacement of 20% of fossil fuels by biodiesel in the coming future [19].
This is driven in part by the potential of biofuels to create a new industry, raise farmer incomes restore degraded lands and promote independence from oil imports hence mitigating climate change [20]. For example, in literature, surveyed India is among the countries that have adopted Jatropa as a second-generation biofuel source of feedstock. However, in literature reviewed, there is no data on, water, pruning, and plant response to fertilizers hence varied planting management practices [21-23].
In biofuels, the main aim has been to identify methods of production by selecting appropriate feedstock, use of efficient conversion technologies and disposal of the product in this case the biofuels. The end product of any process in the world has become critical due to environmental issues and impact products bring, such biofuels production on the immediate environment [24]. One of the leading challenges in the energy sector is the promotion of biofuels compared to fossil fuels through arguments of sustainability i.e. economic environment and social aspects combined [25]. Sustainable development is a common term appearing in the global agenda of development since 1987. It is defined as development tailored to meet the needs of the present generations without compromising on the future generational needs in meeting today’s needs [26, 27]. Sustainability requires that all environmental factors of impact be assessed in each of their phases of the biofuels chain such as (i) Production and collection of feedstock, (ii) Feedstock processing, (iii) Conversion to biofuels, and (iv) Distribution of the end product [28, 29].
The second point is sustainability, which according to literature surveyed is difficult to conduct and evaluate sustainably. For example due to a great number of competing interests interfacing factors are weighted different by stakeholders hence disagreements and lack of common approach [28, 29]. Measuring sustainability of the biofuels industry is a complex issue especially considering the diverse range of biofuel feedstock, pathways, variation in stakeholder’s interests and competing interests. Literature surveyed thus advocates for establishment of other indicators, which will enable assessment of sustainability of bioenergy systems. This should apply to small, large and local infrastructure acceptable to all stakeholder and in diversity [30-32]. Figure 2 showing the interrelated pillars of sustainability between society, the environment and the economy.
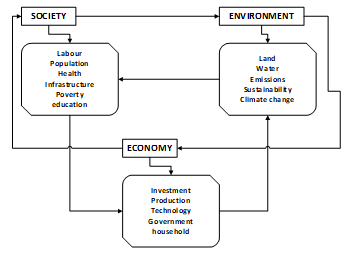 Figure 2: Interrelated pillars of sustainability adapted from [25]
Figure 2: Interrelated pillars of sustainability adapted from [25]
2.1. First Generation Biofuels
First generation biofuels are derived from food crop feedstocks usually used as staple food. However, today this generation is vilified as the source of food insecurity and rising inflation in developing countries. Nevertheless, it is important to note even if it is alleged first generation cause increment in food prices and inflation its total influence is minimal [33]. First generation biofuels ( otherwise called conventional biofuels) use three different technologies commercially for biodiesel, bioethanol and biogas [34, 35]. These commercial fuels are utilized as solid, gaseous or as liquid fuels. To add value these fuels are upgraded to high-density energy fuels such as charcoal, liquid fuels such as biodiesel, and bioethanol or gaseous fuels such as hydrogen, natural gas or biogas [33]. Table 2 shows the characteristics and demerits of first-generation biofuels.
As a first generation, conventional fuel biodiesel is produced through transesterification of vegetable oil, residual oils and animal fats as alternatives for petroleum diesel with slight engine modifications. In the processing triglycerides are chemically reacted with alcohol in the presence of a catalyst or enzyme. This process generates biodiesel and glycerol [36, 37]. On the other hand, bioethanol are produced through a Biocatalytic fermentation of sugar or starch as ether (ETBE) which is used as a blend with gasoline. the gaseous category biogas a mixture of methane and carbon dioxide is processed through anaerobic digestion of organic materials [38, 39].
Table 2: Shows characteristics and demerits of first generation biofuels
| Characteristic | First generation biofuel | References |
| Completion with food crops | Made from edible oil and starch feedstock | [40] |
| Land footprint | Requires arable land | [41] |
| Conversion to biofuels | Easy conversion | [42] |
| Water footprint | Potable water is required for cultivation | [43, 44] |
| Environmentally friendly | Using pesticides and chemical fertilizers | [45] |
| Commercialization | Commercially produced | [40] |
| Sustainability | Not sustainable in using natural resources such as water and land | [46-48] |
| Nutrient requirement | Chemical Fertilizers as main nutrients | [49] |
| Harvesting | Done by hand or machine | [50] |
| Regulation | Clear fair regulation | [40, 51] |
| Financial input | Low capital investment | [40] |
| Environmental condition | Temperature and humidity must be suitable | [52] |
Despite their acceptance first generation, fuels have demerits, which have made their global appeal and wide commercial application difficult. For example, their overdependence on agricultural food crops initiates a heavy social debate on food vs fuel. Hence, their commercialization and adoption threaten food security while inflating food prices in developed countries. In semi-arid areas, production of biofuels would be too costly and limited with determined prices considered as non-competitive to conventional fuels. Figure 3 is showing different biofuel families and their available feedstocks sources.
2.2. Second Generation Biofuels
Second generation biofuels family is also called advanced biofuels. These fuels are also purported to be produced sustainably in a truly carbon neutral environment in terms of CO2 concentration. The source of feedstocks of these fuels is lignocellulosic biomass, non-food crops, agricultural and forest residues and industrial wastes. The production of second generation is done by utilizing physical, thermochemical and biochemical technologies processing [55, 56]. Using these processes involves use of pre-treatment steps to facilitate the conversion process. Here properties of biomass are technically analysed such as size, moisture and density before treatment and processing is commenced [57].
In physical processing techniques in literature reviewed, the commonly applied techniques are briquetting, pelletizing and fibre extraction. These techniques enhance and convert loose biomass into high density solidified blocks of energy. Pelleting does the same thing pressure does to compact raw fibre particles of biomass into high density. On the other hand in fibre extraction fibre is removed from biomass residues and utilized as sources of energy fuel for heating [55, 58].
Under thermochemical processes for production of biofuels are found pyrolysis, gasification liquefaction and direct biomass combustion. Thermochemical liquid fuel processing uses thermal decomposition with chemical reformation. This involves heating biomass under the influence of different oxygen concentration leading to conversion of all organic components [39]. Pyrolysis is a slow or fast process depending on the existing operating conditions but in the absence of air [59, 60]. The former favours solid fuel production compared to the latter which is good for liquid fuel production (bio-oils) and gaseous biofuel production [61, 62].
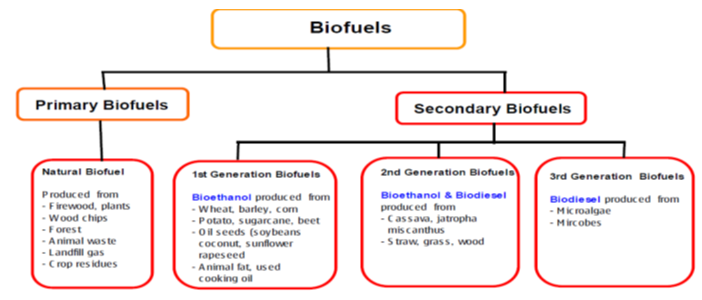 Figure 3. showing different biofuel families and their feedstocks [53, 54]
Figure 3. showing different biofuel families and their feedstocks [53, 54]
As a method in second-generation biofuels, gasification coverts biomass into combustible gaseous fuel mixtures also known as syngas. Through partial oxidation of biomass at elevated temperatures of 800 ℃ to 1400 ℃ syngas is produced. The medium for gasification can be air oxygen of steam [55, 60]. However gasification is still struggling with challenges of operational and downstream gas utilization problems [63]. The driver of gasification has been the versatility of the gases produced during the process. Reaction of the gasification and the feedstocks of solid carbon structure forms carbon dioxide or hydrocarbons. Nevertheless, as said earlier the gasfying agent has a very critical role in influencing the final product of the process. For example when steam is used as an agent of processing reaction temperatures are lowered to 600℃ [39]. Table 3 is showing the principle components of a gasification process.
Table 3: is showing the principle components of a gasification process adapted from [63]
| Target compounds | CO, H2, CH4, C2Hx, C3Hx |
| ‘Inert’ (non-combustible) compounds | CO2, H2O, (N2) |
| Trace contaminants | NH3, HCN, other organic nitrogen compounds |
| H2S, COS, CS2, other organic sulfur compounds | |
| HCl, NaCl, and KCl aerosols | |
| Condensable fraction | Benzene, toluene, and xylene (BTX), tar, hetero-organics, (water) |
| Particles | Ash, mineral matter/salts, char, aerosols |
During transformation (conversion) of solid fuel into gaseous components by gasification a number of reactions with intermediate reactions, take place. It is a complex network of reactions; influenced by feedstock sources, and properties, residence time, reactor design temperature gasifying agent and pressure. In this literature review, only a few examples of main reactions are presented in chemical equations 1 to 9. However, the references provided here would be useful to the reader for a detailed study and understanding [39, 64-66]. Table 4 is third generation biofuels characteristics and references.
Chemical reactions with molecular oxygen, which are exothermic in nature (combustion reactions).
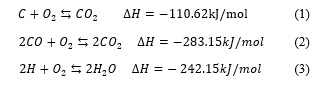 Carbon dioxide and hydrocarbon/CO2 reaction
Carbon dioxide and hydrocarbon/CO2 reaction
Boudouard reaction:
![]() Hydrocarbon and steam reaction:
Hydrocarbon and steam reaction:
![]() Water gas-shift reaction (homogeneous water-gas shift conversion)
Water gas-shift reaction (homogeneous water-gas shift conversion)
![]() Hydrogen reactions in gasification
Hydrogen reactions in gasification
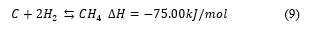 2.3. Third Generation Biofuels
2.3. Third Generation Biofuels
The third generation biofuel family comprises of fuels produced from microalgae feedstocks. This generation is currently under research and development as alternative renewable energy sources for biofuel processing and production. This family has been largely been fronted as a solution to overturning the demerits of the first generation and second generation biofuels [55, 68]. Majority of these fuels exist in laboratories under research and development with a few small scale enterprises producing algae oil [69]. The microalgae group of feedstock consists of microalgae, macroalgae (seaweed) cyanobacteria (blue-green algae) [70]. Microalgae consists of 72000 different species and as many as 800000 in fresh water or salty water [69, 71].
Table 4: Shows characteristics and references of third generation biofuels
| Generational Characteristics | References |
| Eliminates food-energy conflict | [40] |
| Contaminated and Non-arable land used for cultivation | [41] |
| Easy conversion due to increased hydrolysis and/or fermentation efficiency | [42] |
| Waste, saline and non-potable water also can be used | |
| Merits (CO2 fixation, waste water treatment, reduced cost of fertilizer) demerits (ecological concerns on marine eutrophication ) | [43, 44] |
| Poor biomass production for commercialization | [45] |
| favorably poor economically | [40] |
| Large quantities of carbon and nitrogen required. Solar energy is only available at daytime. Nutrients recycling possible | [46-48] |
| Harvesting of microalgae is expensive, and complicated | [49] |
| Lack of regulation for marine cultivation | [50] |
| initial large scale cultivation costs too high | [40, 51] |
| cultivation in harsh environmental condition such high pH, salinity and light intensities possible | [52, 67] |
The rise of algae as an alternative biodiesel feedstock is due to its rapid growth, increased harvest cycle in days compared to months or years. Microalgae need few nutrients for maximum growth and can thrive in severe poor conditions while giving high and increased output per acre ranging from 15 to 300 times more compared to food crops acreage [72, 73]. Algae oil can also be used as feedstock to provide high value products such as ethanol, butanol, biodiesel, jet fuel syngas, and bio-oil chemical feedstocks such as hydrogen and farm fertilizers [53, 70].
Another important factor with algal biofuel is the lack of competition with food crops or feed crops, yet algae is used to reclaim agricultural wastelands. This seems to obviate and free land use while reducing energy versus food competition. This elevates this family against the first two-biofuel families. However a further research and study aimed at improving algae production methods especially the plant energy content yield and sustainability is required [54]. Table 5 shows different oil contents for a variety of microalgae species.
Table 5: Showing different Oil contents for a variety of microalgae adapted from [41]
| Microalga Oil content | Percentage in dry weight (%) |
| Botryococcus braunii | 25–75 |
| Chlorella sp. | 28–32 |
| Crypthecodinium cohnii | 20 |
| Cylindrotheca sp. | 16–37 |
| Dunaliella primolecta | 23 |
| Isochrysis sp. | 25–33 |
| Monallanthus salina | 20 |
| Nannochloris sp. | 20–35 |
| Nannochloropsis sp. | 31–68 |
| Neochloris oleoabundans | 35–54 |
| Nitzschia sp. | 45–47 |
| Phaeodactylum tricornutum | 20–30 |
| Schizochytrium sp. | 50–77 |
| Tetraselmis sueica | 15–23 |
2.4. Fourth Generation Biofuels Sources and Beyond
The birth of fourth generation biofuel has been propagated by the need arising from environmental dilemmas. These dilemmas challenge our human capacity for sustainable solutions to protect nature for our own existence and posterity. For example, the need to protect water sources, agricultural lands for sustainable food production, reduction of GHG, the protection of atmospheric air and weaning overreliance from fossil fuels as the only sources of primary energy supply [74-79]. Table 6 Showing characteristics and references of fourth generation biofuels.
Table 6: Characteristics and references of fourth generation biofuels
| Generational Characteristics | References |
| Eliminates the food-energy conflict | [40] |
| Contaminated and Non-arable land used for cultivation | [41] |
| Increased hydrolysis and/or fermentation efficiency | [42] |
| Waste, saline and non-potable water also can be used | [43, 44] |
| Offers Medium (CO2 fixation, waste water treatment) but releases GM organisms | [45] |
| Produces less biomass for commercialization | [40] |
| Leaking of GMO to environment pausing ecological risks | [46-48] |
| Large carbon and nitrogen required. (However, Solar energy is daytime). Requires Nutrients recycling in the process | [49] |
| Harvesting of microalgae is expensive, and complicated | [50] |
| No regulation for marine cultivation but strict regulation is required with GM algae | [40, 51] |
| Initial cost for large scale cultivation is expensive | [40] |
| Cultivation in harsh environmental condition possible (such as high pH, salinity high light intensities) | [52, 67] |
Fourth generation biofuels are oils therefore produced from genetically modified feedstocks able to consume more CO2 from the atmosphere than what they will produce during the combustion phase as fuels [80]. Therefore fourth generation fuel utilizes the existing platform technologies such as pyrolysis, gasification, solar to fuel and genetic manipulation of organism’s genetic order. The fourth-generation family is referred to as smart fuels based on the conversion of vegoil and biodiesel to biogasoline using advanced technology.
Despite large-scale production and efforts to try to commercialize this family progress has not been sufficient. This has been due to lack of sufficient biomass, increased production costs and set-up, environment and human health concerns as fewer feasibility studies have been completed [40]. Table 7 is showing health and environmental effects of fourth generation biofuels. While Figure 4 is showing main steps of algal biomass technologies in carbon fixation.
Table 7: The health- and environment-related risk of GM algae Human health [81, 82]
| Topic Risk contribution | Risk contribution | Effect | References |
| Allergies
|
Human health
|
Dermal, ingestive, respiratory exposure | [82-86] |
| Antibiotic resistance
|
Human health
|
Reducing the effectiveness of medical treatments | [84-86] |
| Carcinogens | Human health
|
Carcinogenic residues | [82] |
| Pathogenicity or toxicity | Environment | Pathogenicity of some strain to human; toxic blooms; chemical transfer; toxic residues | [82, 87, 88] |
| Change or depletion of the environment | Environment | Removal of nutrients from ecosystem; reducing biodiversity of the flora and fauna
|
[89, 90] |
| Competition with native species
|
Environment | Outcompete native organisms; changing aquatic ecosystems
|
[91, 92] |
| Horizontal gene transfer | Environment | Transfer of genetic organisms | [93, 94] |
| Pathogenicity or toxicity
|
Environment | Pathogenicity of some strain to human; algal blooms; generating genetic–related toxins
|
[95] |
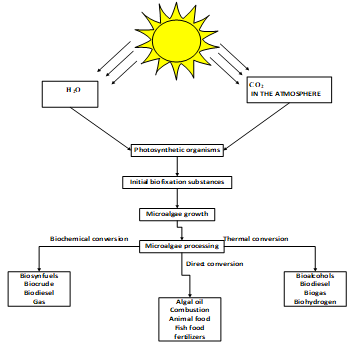 Figure 4: Carbon dioxide fixation and main steps of algal biomass technologies adapted [80]
Figure 4: Carbon dioxide fixation and main steps of algal biomass technologies adapted [80]
GM crops have been with us since 1996 as sources of 3rd and 4th generation biofuels and have increased in their global acreage. For example soybeans, maize and rapeseed occupy 73.3 1013, 46.8 1013, and 7 103respectively of all global land mass area under cultivation [96, 97]. Nevertheless, in order to increase fourth generation biomass production of biofuels algae is heavily GM sourced. Which is achieved by improving areas of the micro-organism using genetic material engineering manipulation. For example, the following areas are mentioned in literature to engineer increased microalgal biofuel production. (i) Improvement of photosynthetic efficiency, (ii) Increasing light penetration by using the truncated chlorophyl antenna and (iii) Reduction of photo-inhibition.
Literature is averse with these developments in the literature reviewed and presented here such as [98-101]. The development in fourth generation biofuels also aims at providing new economic development opportunities in remote and suburban areas of developing countries. These opportunities include reduction of emission to zero for both air pollutants and GHG [102, 103].
Microalgae form a large group of eukaryotes and cyanobacteria and have a wide range of compositional characteristics which include; [104] Food reservation, Photosynthetic pigments, Cell wall chemistry and reproduction. However out of the many species of microalgae only Bacillanophyceae (diatoms), Eustigmatophyte, Chlorophyceae and Chrysophyceae are potential sources of biofuel production [105]. Microalgae have high adaptability in extreme environmental conditions such as high salinity, drought, photo oxidation, osmotic pressure, temperature, anaerobiosis and ultraviolet (UV) radiation [106]. Their main nutrients are nitrogen and phosphorous which accounts for 10% to 20% of its biomass [107, 108].
3. Biodiesel Feedstock Sources, Production, and Processing Techniques
3.1. Introduction to Edible Vegetable Oil and traditional Feedstocks
The production of biofuels such as biodiesel is becoming convenient as alternative energy. The use of biofuel such as biodiesel reduces GHG and provides opportunities for local and regional development in remote areas. This is made feasible considering the number of feedstocks sources. Biodiesel feedstock types differ from country to country depending on the geographical locations and their development [109, 110]. Globally more than 350 oil bearing crops have been identified [111] as possible biofuel feedstocks. However, there are many relevant candidates for biofuel production f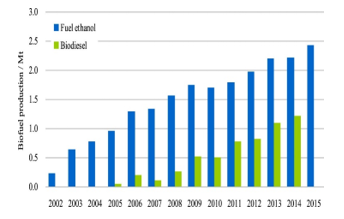 eedstocks adding to the list but are non-plant based. Figure 5 is showing the growth in vegetable oil production in China from 2003 to 2015.
eedstocks adding to the list but are non-plant based. Figure 5 is showing the growth in vegetable oil production in China from 2003 to 2015.
Figure 5: China’s biofuel production over the past decade [112]
The advance in experimental biodiesel production from theses feedstocks has led to a waste to energy revolution. Biodiesel production from readily available feedstock considered waste candidates for dumping and landfill [113]. This has encouraged value addition and co-product markets while contributing to diversification of the biofuel industry [114]. Nevertheless, since each feedstock is different from the other the conversion and processing techniques vary but the basic process of production remains the same. The wide range of feedstocks availability plays a significant role in promoting the biofuel industry.
The availability of feedstocks is influenced by regional climate, geographical location, local soil characteristics and general agricultural practices of a region or country [115]. In sources of biofuel development production and processing, only sunflower, cottonseed, safflower, rapeseed and peanuts are considered compared to two, corn and sugarcane for bioethanol used in gasoline engines;. However, it is important to mention here that non-edible oil plants such as Karanja, rubber seed, tallow oil and microalgae, Jatropa and neem seeds are also gaining acceptance as alternative sources of biofuel. In the last decade, non-edible feedstocks have been extensively studied in literature surveyed such as [77, 116-118].
Among plant-based feedstocks palm oil, soybean and rapeseed account for almost 80 % of global feedstock for biofuel production as shown in Table 8. To qualify as a feedstock for biodiesel production the oil percentage and yield are important factors for consideration [119-122]. Nevertheless quality, availability, physicochemical properties, composition and production costs are some of other critical factors of feedstock determination and acceptability [119]. Feedstock prices account for more than 80% of the cost hence selecting and quality feedstock is vital to ensure low production costs [120, 123]. Since feedstocks are impacted by oil percentage yield, these factors are critical and need consideration. Figure 6 is showing total global vegetable oil production and production contribution of each source from 2013 to 2018. While Table 8 shows major vegetable oil producers and their main sources of feedstock respectively. On the other hand, Figure 7 is showing, international vegetable oil prices from 2000 to 2014.
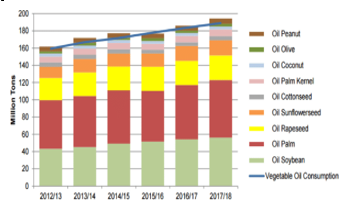 Figure 6: Global vegetable oil production and consumption [124]
Figure 6: Global vegetable oil production and consumption [124]
3.2. Soybean Biodiesel Feedstocks
Soybean comes from the Glycinemax L. as a legume with an annual cycle from the Fabaceae family [130]. United states of America, Brazil and Argentina are the leading producers accounting for 80% of all global production [131]. However, china is increasing production and catching up, hence expanding its role in the near future considerably. It is important also to note that china imports almost 1/3 of the world’s production of soybean. This accounts for 62% of all global soybean trade [132]. Soybean as a grain contains 14% to 17% oil, 33% to 40% protein [133]. In the period of 2013 to 2015 soybean was responsible for over 65% of the global supply of protein supplements and feeds [134]. Soybean meal is the extraction obtained from the oil extraction process and one of the most important source of human and animal protein [135-138].
Table 8: Shows major vegetable oil producers and their main source of feedstock [12, 125-129]
| Country | Production (1000t) | Main feedstock |
| USA | 4.150 | Soybean (53%) |
| Brazil | 3.000 | Soybean (77%) |
| Germany | 3.000 | Rapeseed (>50%) |
| Indonesia | 2.750 | Palm oil (100%) |
| Argentina | 2.550 | Soybean (100%) |
| France | 1.850 | Rapeseed (>50%) |
| Thailand | 1.050 | Palm oil (77%) |
| Total Europe | 10.200 | – |
| Total world | 26.150 | – |
In addition soybean extracts are used in the textile and plastic industry as molds, glue or adhesives for laminated paper and wood [139]. Soybean is also the main raw material in the vegetable oil industry, whose production by 2012 exceeded 40 million tonnes representing 25% of global production of vegetable oils [140]. Environmentally soybean compared to fossil energy consumption over the biofuel life ranges from 2 MJ/MJ-1 to 8.5 MJ/MJ-1 [141-143]. On the other hand, comparatively its GHG life cycle varies from 8 gCO2e MJ-1 to 42 gCO2e MJ-1 of biodiesel [143]. However, in the literature surveyed only one researcher reported higher values of 50 gCO2e MJ-1 [142]. This was attributed to the assumption adopted during life cycle analysis hence the difference in the values of the findings [114].
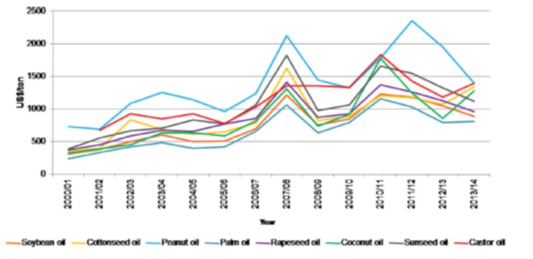 Figure 7: International prices of vegetable oils from 2000 to 2014
Figure 7: International prices of vegetable oils from 2000 to 2014
3.3. Rapeseed Feedstocks for Biodiesel Production
Known scientifically as Brassica hapus L., rapeseed is also known as colza and one of the most cultivated crops globally only beaten in acreage by soybean [140]. Its main geographical origin is the Mediterranean area and northern Europe. Among the leading global producers of rapeseed include China, Germany Canada, India and France accounting for 65% of global production [140]. Table 9 shows Oil yield for major non-edible and edible oil sources and feedstocks.
In Europe rapeseed is the main plant biofuel source accounting for 55% of the total European production of biofuel feedstocks and 68% of global feedstock production in 2016. However, its market share has been slowly decreasing as more recycled oil and alternative feedstocks eat away its market share since 2016 [144]. Other usefulness of rapeseed is in the protein range and food supplements, rapeseed can be used as an animal feed for pigs, cattle, sheep and poultry [145, 146].
Table 9: Oil yield for major non-edible and edible oil sources
| Type of oil source (feedstock
|
(kg oil/ha)
|
Oil yield
(Wt %) |
Prices
(USD/ton)
|
Literature references |
| Jatropha | 1590 | Seed:35–40
kernel:50–60
|
370 | [5,6] |
| Rubber seed | 80–120 | 40–50 | 1250 | [7][147] |
| Castor | 1188 | 53 | 1600 | [5,9][148] |
| Pongamia pinnata | 225–2250 | 30–40 | 286 | [8],[149], [150] |
| Sea mango | N/A | 54 | N/A | [9] |
| Edible oil
Soybean |
375 | 20 | 684
|
[5,11] |
| Palm | 5000 | 20 | 600
|
[5,12 |
| Rapeseed | 1000 | 37–50 | 683 | [5,13 |
Rapeseed meal is known to contain 34% to 38% protein with an oil range of 34% to 40% [151]. However rapeseed has an unpleasant flavor due to glucosinolates which sometimes lead to toxicity in combination with the enicic acid [152]. This is one of the major drawbacks especially on cost reduction in related industries even though utilization can be undertaken by processing [153]. As a source of feedstock rapeseed has low levels of saturated fats coupled to high levels of monosaturated fats with omega 3 and 6 making it a healthy source for human consumption [96]. As a plant feedstock rapeseed ensures a steady acreage yield at stable production over a range of time [154] while allowing intercropping. Compared to soybean, and palm oil, rapeseed performs well in emission studies for example it reduces smoke, PM, and UHC [155]. Environmentally, life cycle studies report a wide range of mixed results. Nevertheless it reduces GHG emissions from 85% to 40% and fossil energy use from 83% to 43% compared to petroleum fuels [156].
3.4. Palm Oil Feed Stocks
Palm originate in West Africa and is used as the main feedstock for palm oil production. Palm oil plant has a life cycle of 26 years [157]. Palm plant as a feedstock generates co-products of 20% to 21% oil, 17% kernel oil, 3.5% palm kernel cake, 22% to 23% empty fruit bunches, 12% to 15% fiber, 5% to 7% shells and 50% liquid POME [158]. Palm oil plants are natural in Central America, north and south. Palm oil is rarely planted commercially due to its low oil content. As such attempts have been made to breed Elaeis OLEIFERA and Elaeis guineensis species to improve disease resistance, palm tree height and increase unsaturated fatty acid [159].
Palm oil as a biofuel feedstock is one of the leading vegetable oils with over 50 million metric tonnes accounting for 30% of global palm oil production in 2013 alone. However, by 2017 the production of palm oil rose to 37.6% (70.3 million metric tonnes) [124]. Nevertheless, as a feedstock only 10% of its global production goes to bioenergy and biofuels [160]. The leading producers of palm oil are Indonesia and Malaysia which control 85% of global production [130]. Palm oil in south east Asia was introduced in Malaysia in 1870 from Singapore [161]. As a feedstock, palm oil has a number of uses considering its byproducts. For example palm oil is used in the food industry as butter, solid fats, cooking oil, industrial oil, baking oil or as a substitute for trans-fat [159, 162].
Additionally, palm oil is applicable in the cleaning, cosmetic, and soap and detergent production. In the chemical industry palm oil is used as a lubricant and in oil production [162]. In waste to energy reform, palm oil shells and fiber can be used in chain production of steam for electricity in co-generation systems. For example, in literature surveyed it was reported that for a palm tree bunch the energy is 300 kWh and 600kg of steam [163, 164]. In literature-surveyed palm oil as a biofuel feedstock has limitations which include; (i) Low supply of seeds, hence poor production [165], (ii) Lack of research in co-product industries and comprehensive economic data due to logistical issues [166], (iii)The long cycle of the palm tree growth [130].
3.5. Cotton Seed Feedstocks
Cotton which is known scientifically as Gosypium hirsutum L., comes from the family of Malvaceae and is grown globally for the production of fiber used in the textile industry [167]. Cotton comprises of 65% seed and 35% fiber [168], oleic acid 15% to 20%, stearic acid 2% to 5% [167], with major fatty acid being palmitic acid at 27.76% while linoleic acid stands at 42.84% [169]. Nevertheless cotton as a feedstock for biofuels has a low oil content at 16% to 23% but with breeding and selection researchers have observed an improvement of 5% in crop oil yield [170].
However, what is of great interest is the cotton meal, which is heavily rich in protein second only to soybean after oil, has been extracted. The cotton meal is mainly used as an animal feed although it has other vital uses such as being a fertilizer, food flour or as a dye in the textile industry and as a biodiesel feedstock [171-174]. Globally cotton is the ninth highest oil producing plant [175]. Despite its low oil content cotton is advanced as a biofuel feedstock due to lower smoke and particulate emissions compared to other feedstocks such as palm oil discussed in earlier subsection [155]. Nevertheless it has limitation which include poor quality oil requiring pre-treatment hence increased cost of biodiesel production when used as a feedstock [114]. Figure 8 is showing a cottonseed oil sample after extraction from cotton seed.
 Figure 8: A sample of cottonseed biodiesel layer of cottonseed oil [167]
Figure 8: A sample of cottonseed biodiesel layer of cottonseed oil [167]
3.6. Sunflower
Known as Helianthus annuus L and a dicotyledon, Its oil content varies from 38% to 50% based on the species of variety employed [176, 177]. Russia is the world leading producer of the sunflower crop with a 21% of the total global landmass, others include Ukraine at 12.97%, Argentina at 11.83%, China at 7.17%, Romania at 6.56%, France at 5.56% India at 4.77%, USA at 4.14% and Spain at 3.09% [178]. Globally sunflower covers an area of 23.7 million hectares with an annual production of 1322 kg/ha on an expected production of 2.3 tonnes to 2.5 tonnes/ha [179].
Beside its potential for oil, sunflower grain produces 250 kgs to 350 kgs of meal shell and 45% to 50% crude protein per tonne of grain [180]. The co-products of sunflower can be utilized for other uses such as in packaging materials, animal feeds, forage silage or as green manure or as a biofuel feedstock [181, 182]. Sunflower in the last decade has gained prominence as a feedstock, for example in Brazil 62000 tonnes were realized in the 2015 to 2016 season. However this was a decrease compared to the previous seasons due to a drop in the acreage under cultivation [183]. The introduction of new crops such as corn saw farmers shift to corn due to low cost of production of inputs and processing [184].
Sunflower is a good feedstock for biofuel due to its drought resistance to cold and heat conditions besides being less likely to be influenced by latitude, longitude or photoperiods [185]. As a feedstock sunflower can be grown in rotation with other plants (intercropping), which offers higher returns to farmers and producers [186]. The main limitations of sunflower as biofuel feedstock include High international market prices [134], besides a cloudy nature as temperatures drop due to its high wax content [187]. Additionally Sunflower contains linoleic, oleic and linoleic acids, which account for almost 70% hindering oxidative properties, which offer stability, hence rapid lipid oxidation of its oil [188-190].
3.7. Jatropa Curcas
This is a plant cultivated almost globally for the production of biofuel although it is a non-edible plant. Jatropa seeds are composed of 37 % shell, and 63% kernel (dry matter) with a protein content of 35% and a 15% of oil [191]. Jatropa has been on the radar of biofuel developers and producers due to a number of factors. These include low production cost, Water stress tolerance, High oil content and yield, Resistance to pests and diseases; Resistance to drought, Good adaptability to semi-arid wastelands, hence reduces competition with food crops for arable land [36, 192].
Other uses of Jatropa as a feedstock include as a cooking fuel, insecticide, soap making, and for medicinal purposes [191]). Additionally Jatropa can be used as an organic fertilizer, livestock feed and a biogas feedstock [193, 194]. On the other hand, the limitations of Jatropa arises: From uncertainties around its seed yield range which is 2 to 12 Mg/ha leading to poor economies of scale for a feedstock [36]. Secondly, even when it seems adaptable to drought during flowering studies have shown it needs watering otherwise the yield drops significantly.
In other words, Jatropa capabilities are not exploitable and applicable simultaneously. This is evidenced by the moisture and nutrients influence on yield [195]. The third limitation is due to its vulnerability to viral infection [36, 196, 197]. The fourth limitation is due to lack of scientific validation regarding the basic ecological and agronomical properties. For example, yield, potential production and costs, and breeding programs have not been identified in the literature reviewed.
4. Introduction to Non-Edible Vegetable Oil and Emerging Feedstocks
The use of non-edible feedstocks for biodiesel production is an answer to the challenges of edible oil as biofuel feedstocks. Non-edible feedstocks are gaining attention, as they are easily available globally in wastelands in unsuited for food crops. This eliminates competition for food, reduction in deforestation rate and co-products. Although there is no direct competition for food versus fuel, Nevertheless, there is indirect competition for land. Among the non-edible plants used for biofuel production include; Cotton (Gosypium hirsutum), Castor (Ricinus curcas), Jatropa (Jatropa curcas), Rubber (Hevea brasiciensis), Mahua (Madhuka indica), Ethiopian mustard (Brassica carinata), Castannola (Terminalia catappa) [198-202]. Figure 9 is showing Waste cooking oil production based on country and global contribution.
In India, there are two major species of the genus, Madhuka longifolia and Madhuka indica, on the other hand rubber seed tree is mainly in Indonesia, Malaysia, Liberia, India, Sri-lanka, Sarawak and Thailand. Rubber as feedstock contains seed kernels of 40 % to 50% brown oil [203]. Another non-edible oil biofuel source is neem (Azadirachta Indica) a natural plant of the Indian subcontinent and commercially grown in India, Bharma, and surrounding regions. Jojoba oil is another non-edible oil a shrub in southern Arizona. Therefore, in Literature reviewed non-edible oil studies as alternative biofuel feedstocks fill many researcher reports such as [44, 201, 204-207].
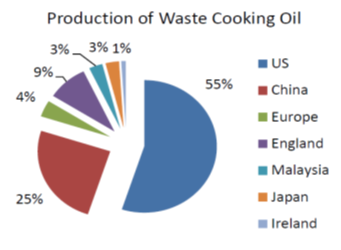 Figure 9: Waste cooking oil production based on country [10, 14-15]
Figure 9: Waste cooking oil production based on country [10, 14-15]
4.1. Waste Cooking Oil Feedstocks
Waste cooking oil is also known as yellow grease due to the fact that most waste cooking oil FFA content is 8% to 12% wt. Waste cooking oil offers a high potential as a feedstock to biofuel production due to low cost. As modernization grows and more people shift to urban lifestyles the total quantity of waste cooking oil has been growing since 2008. Waste cooking oil can be collected from households and hotels and restaurants, fast food outlets with heavy use of frying activities [208]. The disposal oil from these activities is problematic as it contaminates ground water. However, their cooking oil sources differ greatly since their base material are plant lipids like corn, margarine, coconut oil, palm oil, olive oil, soybean oil, grape seed oil and canola oil. The most commonly used material for vegetable oil is palm oil [209].
The use of WCO as a biofuel feedstock does not come at the expense food versus energy or land resources. Instead it’s a sustainable use of resources which reduces adverse effects of water pollution and blockage of water and drainage sewage system [210]. Presently WCO has been heavily utilized in soap manufacturing, although the soap produced is of poor quality hence its utilization has been low [211]. The main objective of using WCO is to transform it by reducing its viscosity to values close to diesel oil. Globally over 15 million tonnes of waste cooking oil are produced annually and if converted it can meet and satisfy the world demand of a biofuel feedstock [212]. Production of WCO oil as a biodiesel can contribute to a saving 21% in crude and a 96% energy saving [213].
Non-edible vegetable have potential to substitute a global fraction of petroleum diesel [214]. Plant oil feedstock when processed to biodiesel reduce particulate and sulfur emission and aromatic compounds [215] compared to petroleum diesel [216]. In 2009, the production of FAME waste oil was 11 tonnes with a demand increase of 3.5 metric tonnes/year. However, the production of waste oil in Europe, north America and in selected Asian countries such as China combined had a production of 16.6 metric tonnes [217, 218].
For example in 2014, China alone had a total WCO production of 1.8Mtonnes [112], pushing China to 3.4 Mtoe hence accounting for 2.9 % of global biofuel production, distributed in 50 plants. In other words, currently, the main leading feedstock of biofuel production in China is waste oil. However its main limitation is waste oil increased prices due to rapid development of the biofuel industry in the last decade [219].
The use of waste cooking oil has a number of challenges due to FFA and presence of high moisture, which make it hard for transesterification. Although chemical and physical properties of waste cooking oil are similar to fresh edible oil they differ from source to source [217]. For example, the water content and FFA in WCO compared to fresh edible is higher due to the frying process. During frying edible oil undergoes higher heating temperatures of 160 ℃ to 200℃ for a long and a sustained period.
As a result, increased viscosity specific, specific heat, surface tension, colour and fat formation occur. Hence reactions of thermolytic oxidative and hydrolytic nature are observed [220]. Nevertheless, current research centres in operation and management of waste to energy with the focus on the following two key areas. (i) The supply chain incentive, and (ii) regulation policies for WCO to energy.
There is a growing importance on the use of subsidies as a measure of addressing issues of waste to energy based on the model dynamics [221-229]. Regulation policies of waste cooking oil to energy report illegal transaction in waste cooking oil utilized as barriers to expanding WCO utilization. This need increases a requirement of standard inspection and regulations with dedicated infrastructure to help in recycling [223, 230, 231]. For example, in America in south California and north western Mexico [232].
4.2. Animal Fats Feedstocks
Tallow is the most common commercially available feedstock from animals for the production of biofuels [233]. The production of meat in the last decade increased significantly to 237.7 million tonnes in 2010 represented by 42.7%, 33.4% and 23.9% for pork, poultry and beef. Nevertheless, the projected growth of these resources increased steadily and now stand at 266909000 million tonnes annually [124]. This corresponds to a representation of 39.76%, 37.3% and 22.97% for pork, chicken and beef. However lard and chicken fat [234] are also commonly used in addition to insects and all other high fat containing animals.
The main reason for use of animal fats as feedstock source is due to their cheap and low prices, hence providing an economical option for biodiesel production [201, 233]. For example, since 2013 the prices of animals based fats has been $ 0.4 to $ 0.5/litre compared to vegetable oil at $0.6 to $0.8 [201]. In the world today 90% of feedstocks for biodiesel production originate from animal fats and greases compared to the USA at 8% to 10% [235]. In other words from this report there is an observed dynamic animal protein and being expressive especially in the poultry production which shows an annual growth of 4% to 5% in the last decade [236].
Animal fats are characterized by a high content of saturated fatty acids and as biological lipid materials they are composed of TAGs and less of di (DAGs), and mono-acylglycerols (MAGs). Animal fats and greases tend to be solid at room temperature compared to liquid oil of plant origin. This is due to their high content of SFAs [233]. Tallow is a waste final product generated in slaughterhouses and meat processing facilities, whose major composition is myristic, palmitic and stearic acids with tallow and pork lard composition of 40% SFA.
However in the literature surveyed the composition figure is higher for tallow at 45.6%, mutton tallow at 61.1%, lard at 39.3% and chicken fat at 32% [207]. Saturated fatty acids present increased demerits on the physical and chemical properties of biofuels. For example fatty acids cause poor cold properties while unsaturated animal fat content offers advantages of high cetane number, oxidation stability and high calorific value [234]. Thus considering the composition of animals fats as a source of biofuel requires synthesis at elevated temperatures compared to processing vegetable oil [237]. Animal fat greases are classified into two types as reported in literature. This classification is based on the level of FFAs,[207] such as Yellow greases with FFAs of ≤15% w/w and Brown greases with FFAs >15%w/w.
In theory animal fats are thought to contribute to oxidative stability for biodiesel, due to the lack of polysaturated fatty acids such as linoleic and linolenic commonly found in vegetable oil [238]. However comparatively in real-life, animal fats are unstable due to lack of anti-oxidants in their structure. Hence use of animal fats and greases eliminates the need for disposal and result in utilization to the supply of biofuels [239]. Like all feedstock, animal fats have limitations, for example, animal fats contain phospholipid or gums, which are insoluble in water.
These precipitates can plug fuel filters and render them ineffective. Secondly animal fats oil biodiesel deactivates exhaust pre-treatment devices in diesel vehicles [238]. Thirdly is the problem of the presence of high sulfur content mainly from sulfur containing amino acids traced from animal feeds [240]. Since animal fats are highly viscous and solid at ambient temperatures due to unsaturated fatty acids. This leads to poor atomization properties, hence incomplete combustion, while increases emissions of pollutants and particulate matter [241]
4.3. Algae Oil Feedstocks
The microalgae family contains more than 100000 species which can be utilized for biodiesel production [242]. However the most one with the highest probability of development in literature surveyed are green algae, diatoms and cyanobacteria (blue algae) [243]. Microalgae content is projected to hit 70% of dry matter and a yield of 90 tonnes/ha of cultivation [244].
Algae grow rapidly in different environmental conditions, while utilizing efficient use of water CO2 and nutrients on the water surface [245, 246]. This requires if planted in a pond stirring becomes a necessity to ensure accessibility to CO2 [247]. In addition to their faster growth and high yield content per acre, microalgae oil contains properties identical to petroleum fossil fuel. This is especially true for viscosity, density, flash point and the hydrogen carbon ratio [248].
Use of microalgae as an alternative fuel is being advanced as the fourth-generation biofuel as technology for producing and processing biofuels increases. This will enable its production as a biofuel to be cost effective for large-scale production in the near future. Microalgae compared to land-based plant feedstocks have efficient photosynthetic process in converting and utilizing solar energy into biomass [249]. The main algae, which can be utilized for the production of biofuel, are cyanobacteria as micro or macro algae.
Their sizes determine and influence the production process techniques. For example microalgae produce high oil content but their harvesting is costly due to low efficiency, cell size and low biomass concentration [250, 251]. On the other hand compared to cyanobacteria which are macroalgae the conversion rate into biomass is good but with a complex membrane rapture [252].
In the literature surveyed to produce algae it requires cell growing, separation, and lipid extraction [246]. The main microalgae growing technologies available vary but open pond and closed photobioreactors are commonly utilized [253]. In harvesting microalgae to extract oil physical chemical and enzymatic techniques are employed [254]. Processing of microalgae oil for biofuel production commonly takes the same route of processing and technologies used in vegetable oil and animal fats. It is important to note that fourth generation technologies for production of algal biofuels are still under research and development.
A number of questions remain unanswered. For example, in literature surveyed on algal feedstocks advantages and disadvantages of growing microalgae in fresh and salty water is not available in literature for all types of algae marked for biofuel production. Another factor noticed from literature surveyed is lack of feasibility studies whose data for biofuel microalgae is unavailable. For example, the cost of production for algae is projected at $0.9/kg to $2.55/kg in open pond systems compared to $1.5/kg to $5.5/kg using photobioreactors.
This is despite development in more realistic and appropriate technologies used for algae on commercial scale [243]. The methods utilized in the farming, harvesting and oil extraction for biofuel production still face surmountable difficulties. Nevertheless, microalgal diseases such as contamination are still not clear, although biofuel especially in chain and value addition offer high returns compared to other feedstocks [255]. If technologies are developed for value addition such as on pharmaceutical, nutraceuticals, biodiesel commercialization etc. could increase economic viability of algal feedstocks for production [256].
4.4. Waste Biomass Feedstocks
A number of waste resources arise in line with the diverse human economic and social activities. However, utilization of natural resources such as water, air, soil etc. are being threatened. This solid biomass wastes take many forms either as solids. Although classified as waste they can be reused and turned into energy resources for industrial and domestic purposes [257]. In the current world energy scenario, a number of waste to energy, technologies have emerged. These technologies convert waste biomass into various forms of fuel before utilization as biodiesel [258].
Bio-waste feedstocks differ greatly from primary sources such as coal, in both energy content and physical properties. However compared to coal bio-waste comprise low carbon, high oxygen content, high silica and potassium, less aluminium and iron, low heating values, high moisture, low density per unit of mass and friability [259]. Figure 10 shows the Main waste to energy (WTE) technologies available currently for the utilization of biomass waste.
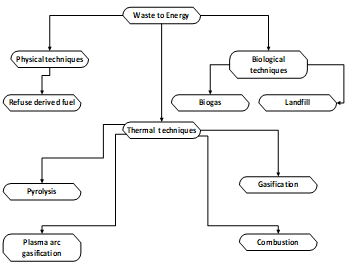 Figure 10: Main waste to energy (WTE) technologies adapted from [259].
Figure 10: Main waste to energy (WTE) technologies adapted from [259].
However, utilization of natural resources such as water, air soil etc. is being threatened and takes many forms either as solids and can be utilized and reused to become energy resources for industrial and domestic use [260]. In the current world energy scenario, a number of wastes to energy technologies have emerged. These technologies convert waste biomass into various forms of fuel before utilization as a biofuel or biodiesel [259]. Depending on the use of the technology so is, the name derived.
For example in the literature surveyed technologies for fuel production are referred as waste to energy technologies (WFT) [259].The waste technologies include the following categories of utilization; Physical methods, Thermal methods and Biological methods. Globally these technologies have grown to nearly 750 facilities with a capacity to process 140 million tonnes of waste annually [261]. Energy from waste can be treated and compressed to solid fuel or converted into biogas, syngas, or combusted to produce heating for steam production in power generation. The gases produced in such cases include methane, CO2, hydrogen, and H3CO or liquid fuels such as ethanol and biodiesel [262].
Biomass to energy has potential feedstocks, which form its main potential line. These include wood, short rotation wood, crop waste, agricultural wastes, short rotation herbaceous crops and animal waste [263]. It is important to note that biomass accounts for 35 % of all energy consumption in developing countries [264, 265]. Nevertheless, biomass utilization carries a huge untapped potential for environmental and energy production, especially agricultural based plants absorbs CO2 during growth and emit it during combustion.
This helps in the recycling of CO2 in the atmosphere hence climate change mitigation [266]. Since biomass feedstocks contain lignocellulosic materials, they inherently produce high content of polymers such as cellulose (C6H10O5)x, hemicellulose (xylas) (C5H8O4)m, lignin (C9H1[267]0O3(OCH3)0.9-1.7]n and sometimes protein. This contributes to renewable energy sources, which are natural, sustainable, inexpensive and eco-friendly feedstocks [260]. Wood biomass thus forms the bulk of waste biomass accounting for 64%, municipal solid waste 24%, agricultural waste 5 % and landfill gases accounting for 5% [268, 269].
4.5. Bioethanol Feedstocks
Bioethanol is one of the leading clean and renewable energy sources in the transportation industry today. Globally bioethanol has seen a growth from 4.8 billion gallons in 2000 to 16 billion gallons in 2007 [270], representing a 30 % increase within the mentioned period. However, current statistic trends paint encouraging prospects, indicating for example that since 2007 with global production of 60 billion litres of bioethanol, by 2017 the figures stood at 143 billion litres annually [271].
Bioethanol has many advantages compared to fossil fuels such as high octane, which prevents knocking in internal combustion engines and high oxygen content, which helps to produce less greenhouse gas effects [272-274]. This advantages Allows direct use of ethanol in the automotive industry for internal combustion SI engines without modification and bioethanol works with other oils as a blending agent.
Currently the USA and Brazil are the leading global bioethanol producers with the two countries combined contributing 75 % to 80 % of the total global production [270, 273]. Using corn grain the USA has 187 bioethanol plants spread across different states to produce ethanol [275]. On the other hand, Brazil produces bioethanol from sugar cane based feedstocks only, compared to the European Union who use wheat and sugar beets. In 2013, Brazil produced 37 billion litres compared to European Union production of 5.785 billion litres of bioethanol and is expected to double its production in the near future.
Due to the reservation on plant-based feedstocks, in future renewable and sustainable feedstocks will dominate energy sources, hence replacing fossil fuels. Bioethanol has been a dominant feature of biofuels, nevertheless technology is moving to microalgae carbohydrates as potential feedstock [276-278]. Microalgae biomass feedstocks contain high contents of carbohydrates, (glycogen, starch, and cellulose) that through fermentation can be converted to sugars for production bioethanol [279, 280].
5. Factors Affecting Biodiesel Production
5.1. Biodiesel Quality
The quality of biodiesel of the feedstock used to produce a biodiesel determines the type of catalyst and process applied to produce FFA for the biodiesel production. Nevertheless, the biodiesel feedstock selection and determination are an important factor, inconsistence in the selection of feedstocks can lead to problems of quality and over-budget production. Suffice to mention that biodiesel fuels have standards recommended for their production such as ASTM D675 and EN14214. For example, feedstocks with more than FFA>3wt% cannot use homogeneous catalysts like NaOH, KOH or methoxide due to unwanted side reaction.
In order to produce biodiesel commercially the commonly used basic catalysts such as NaOH, KOH, or Methoxide are utilized. In addition, as a general rule acid catalyst are more appropriate for high FFA content feedstocks. On the other hand, homogeneous catalysts require less alcohol; have a shorter reaction time even though they result into complex products. This leads to required product purification compared to heterogeneous catalysed transesterification process. Development in catalysts has ensured use of a wide range of catalysts for biodiesel production such as heterogeneous and homogeneous acids, bases, sugars, lipases ion exchanges resins, zeolites etc. A number of researchers in the literature reviewed mention biodiesel quality as a factor influencing production include [135, 281-286].
5.2. Cost of Biodiesel Feedstocks, Investment and Material
Another factor that influences biodiesel fuel production is the higher cost of production arising from erratic feedstock prices as biodiesels gain widespread application. This is increased by the chemical composition due to their relatively low energy content increased NOx emissions compared to fossil fuels such petroleum diesel [287]. Price determination is an important factor of biodiesel production system and processing.
In every production system of biodiesel, a 50% feedstock price should be the guiding principle of all cost of production. Another factor to consider and is proposed in literature reviewed is price fluctuation especially when promoted through government policy shifts in relation to subsidies and tax incentives [8, 288, 289]. In other words when alternative sources are promoted there is diversification and stock piling to create demand and stabilize stabilization [287].
A number of researchers have reviewed and reported on this concept and can be read in some references provided here such as [239, 290-295]. In all the literature surveyed there is agreement that the higher cost of biofuel production is a major barrier for acceptability and use of biodiesel as an alternative fuel [296, 297].
In literature surveyed, a number of suggestions thus come forward to address this issue. For example, use of cheaper alternatives catalysts Coupled to conversion technologies with lower energy input and faster transesterification reaction [201, 296, 298-301]. Another commonly suggested solution in literature surveyed is the diversification of feedstock by increasingly moving to material materials, not formerly considered as feedstock [302-304]. Figure 11 shows the effect of government policy and promotion to create demand and stabilize prices on palm compared to petroleum diesel.
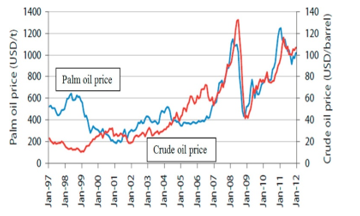 Figure 11: Effect of government promotion on price of feedstock price fluctuation 1997-2012 [305]
Figure 11: Effect of government promotion on price of feedstock price fluctuation 1997-2012 [305]
5.3. Effect of Tax Policy Subsidy and Regulation
In order to empower biofuels and bring them into mainstream economy as usable fuels there is need to implement laws and regulation. These laws should include taxes, policies and subsidies from government as incentives to develop this sector. Particularly in the area of enforcement which mostly needs guidance in order to improve the basic economics of biodiesel.
In the literature surveyed for example, European Union has proposed a renewable energy policy for transport fuels to ensure a viable expansion of the biofuel industry in the near future. As a result, the European Union introduced a blending target in their range of biofuels, which involved hitting a target of 5.75% to 10 % by 2020. Additionally, an amendment to the fuel quality service brought into service a mandatory 6% reduction of GHG by 2020 for transport fuels and non-road and transport engines [306].
Between 2000-2013 biofuels such as ethanol and biodiesel grew exponentially in terms of output from 64 million to 23 billion litters and 0.8 to 14.7 billion litres respectively [307]. For example the USA also crafted a renewable fuel standard program to increase the share of biofuels by 10 % by 2017 and beyond since 2005 [308]. This increment in biofuel production is purely driven by government policy interventions. This is particularly so in the USA where financial incentives are almost guaranteed for producers [309].
Due to policy shift and incentives, a larger share of global biofuel market has been taken from ethanol to biodiesel in the market [310]. In other words, appropriate policies, tax, mandates and incentives can successfully drive growth of biodiesel. However, it is important to note that biodiesel incentives are distortionary in nature as biofuel use uniquely food crops and multiple feedstocks [311].
In other words, in 2006 for example 20 % of all USA corn production went to biofuels compared to ethanol. Although indicating a positive development in the growth of biofuel, this led to increased producer prices between 2003 to 2008 [312-315]. On the issue of regulation and GHG emission studies in literature reviewed, indicate a negative impact in reduction for specific types of crop feedstocks and their processing techniques. For example, this is revealed in studies conducted by researchers such as [316-318].
5.4. Competition with the Food Industry Chain
The increased biofuels production supported by the government policies interferes with food industry chain such as the oleo chemical industry. These industries use the same feedstocks as biofuels hence the rapid growth in the biofuel, which threatens their growth in the future. This is true considering the level of increased feedstock prices yearly. In other words, resource availability will be constrained hence causing a negative impact on the economic value of the food chain [288, 319, 320]. As the Competition for feedstock resources increases with greater incentives to encourage producers meet the demand. This leads to increased demand for resources and with it increased prices for land resources and prices for food [321].
In order to implement a sustainable biodiesel production strategy. The focus should not only be on reduction of GHG emissions but rather a complete package of policies, which support economic and environmental sustainability. However, one notes that the increased biofuel production from land resources and plant feedstocks is the leading cause of deforestation.
In recent trends governments defend their guidelines on implementation of sustainable policies on deforestation as is the case in brazil though it is not sustainable [288]. The second issue on sustainability is related to animal habitat destruction when deforestation is carried in large scale with some of these animals facing extinction [322]. This is due to replacement of virgin forests with plantations of plants such as palm oil. This can led to global warming especially in lower latitude areas while causing overcooling in high latitudes [323]. Use of biofuel in the transport industry when fully running is hoped to stabilize the global carbon cycle although deforestation will negate it. A practical example is in Malaysia where as a consequence GHG emissions increased to about 40 million tonnes of CO2 up from 5 years earlier at 20 million tonnes of CO2 [324, 325].
Another area that is often ignored in literature surveyed is change in land use, even with sustainable programs and policies. This problem is acute and severe in America, Mexico, and brazil where abuse of power forces farmers to sell land and move out leading to large population displacement and demographic changes [320]. However the European union seems to contain the problem by putting requirements for types of land to be used for biofuel production plantations as pointed in a number of literature surveyed such as [326, 327].
5.5. Biomass composition
In biomass composition, the most influencing factor is the carbon to hydrogen ratio. In other words, due to the differences in decomposition temperature for each of the constituents of biomass. These constituents thus undergo decomposition hence varying product yield. For example, in literature surveyed the following are the temperatures of the three main ligno-cellulosic biomasses [328-330]. This include Hemicellulose with a range of 150℃ to 350℃, Cellulose with a range of 275℃ to 350℃ and Lignin with a range of 250℃ to 500℃.
5.6. Particle Size
The particle size and composition, the physical structure and shape have a greater influence on the pyrolysis process products when exposed to heating [331]. For example, fine particles offer less resistance to escaping non-condensate gases and vice versa. This behaviour affects product yield [332]. In other words, size reduction of biomass before product extraction offers greater surface area for mass transfer. This enhances diffusion of the active components within the feedstock [333]. In literature surveyed, most scholars report these factors as variables in optimizing bio-oil production. Among the scholars who take this position, include the following researchers [334-337].
5.7. Effects of Temperature on Pyrolytic Process
Temperature is an essential factor of influence on pyrolytic product yield. Pyrolytic temperature defines the rate of increase from ambient to maximum until the completion of the process. Pyrolysis temperature thus influence composition and product yield and release rate of the constituent gases. Besides these components, the char produced depends on the pyrolysis temperature [336, 338, 339].
In other words, as the temperature of the reactor increases, the carbon content of the pyrolytic char products increases. This is due to the increase in surface area of the char. However beyond 1173k the temperature decreases slightly due to structural ordering and micropore coalescence with increasing temperature for char above 1073k [340, 341]. Increased heating rate results into a decrease in the carbon content while increasing hydrogen and oxygen content of the char [342].
In literature, surveyed pyrolysis takes many forms of processing but four forms of the criteria are critical namely: Slow pyrolysis, which is a carbonization pyrolytic process with a primary goal of producing charcoal and char. This is the oldest form of pyrolysis operating at below <400℃ over an extended period. Fast pyrolysis is tailored for liquid or bio-oils where biomass is subjected to rapid high temperature heating before decomposition begins. The rate of temperature increase can range from 1000℃/s to 10000℃/s. Nevertheless, the peak temperature is maintained between 650℃ to 1000℃. The main features of fast pyrolysis are high heating rate, reaction temperatures of 425℃ to 600℃, short residence time (<3s) and rapid cooling of the gas product.
Flash pyrolysis where rapid heating of biomass occurs in the absence of oxygen at moderate temperatures of 450℃ to 600℃. In other words, the products of both condensable and non-condensable gas leave the reactor unit faster (short residence time) of 30ms to 1500ms [331]. Ultra-rapid pyrolysis, which borders on the extreme fast mixing of biomass with heating and a carrier solid leading into high transfer of heat and rate of heating. Ultra-rapid pyrolysis utilizes temperatures of 1000℃ for gas components compared to 650℃ for liquids in order to minimize product yield [343, 344]
5.8. Effect of Heating Rate Change
It is important to note that the heating rate of biomass particles from rapid to moderate 400℃ to 600℃ leads to high volatile yields by producing more char [345]. Owing to fast volatile material release which causes internal pressure and coalescence of smaller pores leading to increased surface area [346]. A number of scholars have studied this phenomenon and reported on it widely such as [347-349]. Nevertheless in literature surveyed it is widely reported that high heating rates of 900℃, a lower surface area is produced and vice versa [350]. In other words higher heating rates cause high char yield interior temperatures, partial graphitization and curtails development of large surface areas [351].
5.9. Effects of Residence Time
Residence time Space is inversely proportional to space velocity of the reactants in a pyrolytic reactor [352]. In other words, residence time has a greater impact on conversion and product yield in the pyrolysis process. Studies conducted on biomass gasification from kinetic models report positively on this influence of residence time. For example, the following researchers report how conversion increases in the first 20s and their after the chemical reaction slows down as reflected in a number of studies such as [353-356].
6. Biodiesel Production and Processing Techniques
6.1. Introduction to Production and Processing Techniques
Biofuels have increased in demand as the global energy demand grows significantly, although with a requirement for clean fuels [297]. Biofuels are degradable and promising fuels compatible with environment preservation and biodegradable [297]. Although biodiesel is advantageous as a biofuel, its major hindrances are high cost of production, processing and raw materials (feedstock). These costs account for 80% of the total cost of production, making biodiesel more expensive than fossil fuels which it is intended to replace [357]. However, one of the most glaring advantage of biodiesel is its use without modification for diesel-powered engines. Biodiesel use reduces emissions of PM, sulfur, UHC, and carbon monoxide due to a high oxygen content and carbon to hydrogen ratio [358, 359].
In literature surveyed a number of researchers have used many production and processing techniques. However, it is clear from the studies that feedstocks dictate and influence these techniques of production and processing. For example, thermal cracking (pyrolysis), catalytic cracking, Nano-catalytic processing, catalytic hydrocracking, micro-emulsion using solvents and surfactants, transesterification, bio-catalysis processing supercritical production processing.
The following literature references shows extensive work that has been done and reported in modern production techniques as knowledge and skills increase in biofuels; [205, 222, 360-365]. Nevertheless, the number of production methods has been increasing as the material science and biochemical-engineering sections grow. This has brought new and novel concepts as will be seen in the preceding sections of this review.
6.2. Biodiesel Thermal Cracking (pyrolysis)
Thermal cracking’s main role is to decompose, rearrange and combine hydrocarbon molecules through application of heat. In other words, thermal cracking decomposes high molecules- weight hydrocarbon components into lower molecules weight. This makes the products more valuable hydrocarbon derivatives with lower boiling point species [366], such as gas liquids and char. Pyrolysis as a thermochemical decomposition process has similarities or overlaps with other processes such as carbonization, dry distillation, devolatilization, destructive distillation and thermolysis.
Nevertheless thermal cracking (pyrolysis) is similar and identical to gasification process [332]. Gasification is more external with chemical reactions compared to pyrolysis, carried out in low temperature settings of 300℃ to 650℃ or higher temperatures of 800℃ to 1000℃. The main factors, which influence the process of thermal cracking, include feedstock type, residence time, operating temperatures and pressure. Many of this factors in literature reviewed are discussed and their influence on thermal cracking processes. However, these factors are discussed here briefly but further reading can be done from the references provided here in this section. The process of cracking alternative oils mainly vegetable or animal oils takes two main forms as mentioned in the following references [367-369].
Primary cracking where decomposition of triglycerides molecules occurs, forming acid species by breaking C-O bonds of the glycerides and triacyglycerides chain. Secondary cracking which involves degradation of the produced acids in the primary stage while forming hydrocarbons with properties identical or similar to petroleum derivatives. The initial products of pyrolysis are condensable gases and solid char. However, further classification of the gases brings a non-condensable class such as CO, CO2, H2 and CH4, liquid and char. In other words, this processes go through gas phase homogeneous reactions, partly gas solid phases heterogeneous reactions [332].
6.3. Chemical Catalysis Production Technologies
Chemical catalysis is the science of materials, which accelerate chemical reactions without affecting the equilibrium position of the thermodynamic reaction. Under this method which mostly describes use of catalysts both alkali and acid in the transesterification process. Transesterification has become a more popular method due to its versatility and ease of use [370]. This scheme utilizes triglycerides by reacting them with alcohol in the presence of a catalysts to produce biodiesel (FAME) which is a type of biofuel [371]. Once transesterification process is completed it produces glycerol by products of the process [372].
Transesterification process comprises of sequent reversible reactions. The first step is the conversion of triglycerides to form diglycerides. Followed by the conversion of the diglycerides to monocerides and glycerol and producing one methyl, ester molecule. It is important to note that the transesterification process is heavily dependent on external catalysts to perform reactions. For activation catalysts in transesterification takes two main forms either biological or chemical.
The chemical catalysts comprise of both alkali and acid catalysts as homogeneous agents [373, 374]. Namely, Heterogeneous agents’ solid acid or solid alkali, Heterogeneous Nano-catalysts, Supercritical fluids (catalysts). On the other hand, biological catalysts come up through genetic engineering and are mostly preferred. This type of transesterification packs an environmental advantage over all other methods as reported by researchers such as [375-377]. However since it is still under research and development, the cost is prohibitive for commercialization and laboratory use [378].
6.4. Transesterification Biodiesel Production Techniques
Transesterification is a process in which none edible oil chemically react with alcohol. It is an imperative process for the production of biodiesel as it reduces biodiesel viscosity of feedstock oil closer to petroleum diesel viscosity [379]. The catalysts used during transesterification are either acidic (sulphuric acid, hydrochloric acid or phosphoric acid) or could be base catalysts such as NaOH, KOH, carbonates and Alkoxides [380]. Base catalysts (alkaline bases) are suitable for oils with FFAs below 3-wt % [381, 382].
Due to their less damage to equipment and efficiency in production, alkaline catalysts are preferred to acidic catalysts in transesterification [383]. During processing in transesterification, it is assumed that 100kg of glycerol will form a cubic meter of biodiesel [166]. This means a large portion of the by-products of the production of biodiesel causes concern to environmentalists. Hence, a number of studies in literature surveyed have studied on how to utilize glycerol, which is a major by-product of this process. For example, a number of researchers have proposed use of glycerol in the hydrogen reaction reforming processes as aqueous or vapour [384-386].
Utilization of glycerol is hailed as a breakthrough in lowering production costs which make biodiesel fuels expensive [387]. In the production of biodiesel 10 %, w/w is glycerol, which translates to every gallon of biodiesel, produces approximately 1.05 pounds of glycerol. For example in a 30 million gallon production plant per year, glycerol is 11500 tonnes. Therefore presenting an opportunity for new application and value addition processes in the commercial and chemical industry. Nevertheless, this area in research is aging behind in terms of data and experimental work, with few researchers having published reviews. Other uses of glycerol identified in literature surveyed include: Glycerol can be used in the production of animal feeds [388]. Glycerol feedstocks are good for chemicals production for example poly-hydroxyalkanoates (PHA), and docosahexaenoic acid (DHA) [389-391]. Glycerol can be used in the production of lipids for sustainable biodiesel feedstock production [392, 393], and in the manufacture of citric acid through biosynthesis [394, 395].
The commonly used alcohols in transesterification reactions include methanol and ethanol due to their low cost and availability. This reaction of alcoholysis reduces viscosity of nonedible oil converting it into triglycerides esters [396]. In other words, transesterification converts the carboxylic acid esters into carboxylic esters. The critical lipid in transesterification include non-polar lipids, triacylglycerols (TAGs) and free fatty acids (FFAs) [397]. There are two main forms of executing transesterification, either through catalytic transesterification or through non-catalytic transesterification [204, 398].
Nevertheless there are two main challenges in literature surveyed related with these two techniques. For example it takes longer time to process biodiesel and there arises a need for separation of the oil alcohol catalyst and the impurities from saponification in the mixture [399]. Therefore, a number of factors, which affect the transesterification process according to literature, surveyed. This factors include such as Reaction temperature, Ratio of alcohol to vegetable reaction, Catalyst used, the mixing speed (intensity of mixing) and Purity of reactants.
6.5. Emulsion and Microemulsion Biodiesel Production Techniques
Emulsion or micro-emulsion processes upgrade commodity fossil fuels by forming emulsion and micro-emulsion fuels. Micro emulsion also upgrades oil from other sources or feedstocks such as bio-oil without the help of a surfactant [400, 401]. Emulsion also helps in reducing problems associated with stand-alone bio-oils, hence reduced pollutants in emissions [402]. Emulsion as an upgrading of fuel method ensures that all the components of emulsion are utilized as fuel resources compared to other techniques discussed in other sections in this review.
Emulsion is among the key techniques that solves the problem of performance and emissions prevalent in internal combustion engines especially diesel propelled ones. This technique is a solution that relies heavily on modification of fuel so that it reduces or eliminates engine modification and redesign. Globally many countries have fuel mandates specifying quantity and type of biofuel to use although the percentages may vary from country to country [403, 404]. For example water emulsion in diesel fuel is regarded as the most economical and effective method to reduce PM and NOX emissions [405]. Emulsion in diesel fuel extends the combustible limit compared to using non-emulsified diesel fuel. This is due to the reduction of combustion temperature as the water in the mixture has a higher specific heat capacity resulting into secondary atomization as the water droplets explode in the combustion chamber [406-408]. Table 12 showing the properties, droplet size, stability, visual appearance, composition components of emulsion and micro-emulsion.
6.6. Blending and Hybridization
Blending in biodiesel production refers to mixing or combining two or more feedstocks into a final product with superior quality and desired characteristics. The blending process has a significant influence on the product homogeneity, as the biodiesel product is denser than petro-diesel besides their differences in cold flow properties. Blending of fuels depends largely on ambient temperature because in cold weather blending may present with challenges or fail in its objectives. There are two basic methods of blending (i) splash blending (either in a tank or in a truck), (ii) in-line blending which includes sequential blending, ratio blending, hybrid blending or side stream blending [409, 410].
Biodiesel hybridization is a new concept that has come up in the study of biofuels. Hybridization is a chemical process of two or more different feedstocks comingled in varying proportions in the production of a new hybrid fuel possessing different physico-chemical properties. Since fuel properties and the physico-chemical configuration of each feedstock vary from source to source, hybridization improves and enhances these properties.
Therefore, the combination of different feedstocks’ (hybridization) enhances and improves properties of the initial parent stock, by adapting to improved and high attributes. It is important to mention here that both blending and hybridization cab be ex-situ or in-situ (the former means after production while the later means before production of biodiesel). Secondly both hybridization and blending produce fuel blends with intermediate properties able to improve combustion and emission characteristics when applied in internal combustion engines [411].
Table 12: Comparisons between emulsion and Microemulsion
| Properties | Droplet
size |
Stability | Visual
appearance |
Composition | Production | Interfacial
tension |
Energy
input |
Chemical
reagent Cost |
| Emulsion | 1 µm -10µm | Kinetically stable,
thermodynamically unstable |
Translucent,
anisotropic |
Water, oil, small amount of
surfactant,no co-surfactant |
Mechanical agitation,
ultrasound |
Low | High | Low |
| Microemulsion | 1nm -100nm | Thermodynamically stable | Transparent,
isotropic |
Water, oil, large amount of surfactant,
and sometimes co-surfactant |
Produced spontaneously
without extra energy |
Ultralow | Low | High |
Table 13: Potential biodiesel yield from triglyceride feedstocks
| Source | Annual yield, gallons/Acre | Reference |
| Corn | 18-20 | [416, 417] |
| Cotton | 35-45 | [152, 418-421] |
| Soybean | 40-55 | [152, 415, 418, 420, 422] |
| Mustard | 60-140 | [415, 423] |
| Camelina | 60-65 | [423-425] |
| Safflower | 80-85 | [420, 426, 427] |
| Sunflower | 75-105 | [428-430] |
| Canola | 110-145 | [431-433] |
| Rapeseed | 110-130 | [235, 434, 435] |
| Jatropa | 140-200 | [436-439]. |
| Coconut | 250-300 | [434, 440, 441] |
| Palm oil | 400-650 | [294, 442-444] |
| Algae | >5000a | [445-447] |
7. Biodiesel Composition and Physicochemical Properties
7.1. Introduction
There is a renewed and continuous increase in the use of biodiesel globally. The composition of biodiesel plays a critical role in dictating physical and chemical profiles of biodiesel FAME materials. A number of researchers such as [215, 412] have investigated this phenomenon. Greenhouse gases, (GHG), have accelerated this global warming, which has caused climate change. The other factor is due to a growing demand and desire to go green by using sustainable energy sources (renewable).
Another important factor fueling biodiesel research and development is energy security domestically as the demand for liquid fuels and supply grows in a fast-changing landscape. In the last decade, a number of countries have embarked on legislative and regulatory pathways to encourage production and use of biodiesel fuels. For example, in the USA using both prescriptive volumetric requirements and incentives.
The energy independence and security Act (EISA of 2007) required 0.5 million gallons/year for biomass-based biofuels to be increased to 1 billion gallons/year by 2012 a target, which has been surpassed [413]. Although biodiesel fuels have a wide variety of feedstocks depending on the geographical location, however the dominant feedstocks are soybean in the USA, rapeseed in Europe and palm oil in South East Asia [414, 415].
Nevertheless, the list of feedstocks is growing to include non-traditional feedstocks such animal fats and lard, used cooking oil, used engine oil, microalgae, municipal solid biomass, canola, coconut, Jatropa, sunflower, safflower, camelina. Table 13 showing Potential biodiesel yield in gallons per acre from triglyceride feedstocks and their references.
In literature, surveyed biodiesel fuel produced contains different varieties of individual FAME species. Nevertheless, a particular feedstock in the literature surveyed could dominate within a FAME species. FAME and FA are classified according to two categories; the first naming uses the number of carbon atoms in the FA chain. The second naming uses the number of carbon double bonds in the chain [414]. Among the 13 commonly found species in literature surveyed, there are 5 species which are dominant and are majorly derived from vegetable and animal fats and include the following; Palmitic acid (16:0), Stearic acid (18:0), Oleic acid (18:1), Linoleic acid (18:2), Linolenic acid (18:3). Table14 is showing the most commonly found fatty acids in the literature surveyed, their common names, formal names, molecular formula and molecular weight.
In biodiesel, composition FAME produced through transesterification is composed of exclusively of even numbered FA chains. It is important to mention here that FAME composition is not limited to vegetable and animal oil feedstocks but includes also algal derived lipids [448]. However, using the hydro-processing on biodiesel alters the FA chain irrespective of feedstock source to odd numbered FA chains. This is due to the removal of one carbon molecule during production of the biodiesel and has been reported in literature significantly by researchers such as [414, 449].
Table 14: Typical fatty acid (FA) groups in biodiesel
| Common name | Formal name | Abbreviation | Molecular formula | Molecular weight |
| Lauric acid | Dodecanoic acid | 12:0 | C12H24O2 | 200.32 |
| Myristic acid | Tetradecanoic acid | 14:0 | C14H28O2 | 228.38 |
| Myristoleic acid | cis-9-tetradecenoic acid | 14:1 | C14H26O2 | 226.26 |
| Palmitic acid | Hexadecanoic acid | 16:0 | C16H32O2 | 256.43 |
| Palmitoleic acid | cis-9-hexadecanoic acid | 16:1 | C16H30O2 | 254.42 |
| Stearic acid | Octadeconoic acid | 18:0 | C18H36O2 | 284.48 |
| Oleic acid | cis-9-octadecenoic acid | 18:1 | C18H34O2 | 282.47 |
| Linoleic acid | cis-9,12-octadecadienoic acid | 18:2 | C18H32O2 | 280.46 |
| Linolenic acid | cis-9,12,15-octadecatrienoic | 18:3 | C18H30O2 | 278.44 |
| Arachidic acid | Eicosanoic acid | 20:0 | C20H40O2 | 312.54 |
| Gondoic acid | cis-11-eicosanoic | 20:1 | C20H38O2 | 310.53 |
| Behenic acid | Docosanoic acid | 22:0 | C22H44O2 | 340.60 |
| Erucic acid | cis-13-docosenoic acid | 22:1 | C22H42O2 | 338.58 |
Among the commonly found FAME majority are dominated by C18 compounds although a few have lighter compounds C12 such as coconut and palm oil with C16. Feedstocks dominated by C18 have their relative saturation at 18:0, mono-saturated at 18:1 and di-saturated at 18:2. Plant based feedstocks such as rapeseed and canola contain 18:1. Corn and safflower, soybean and sunflower 18:2, Jatropa and yellow grease have similar values 18:1 and 18:2. However, Camelina in literature surveyed contains the highest level at (18:3) while Jatropa has lignocenic acid level of (24:0).
The physicochemical properties of biodiesel are determined by the compositional profile. The physicochemical properties of biodiesel vary substantially as with feedstock source [450, 451]. Due to higher oxygen, content (11% or more) biodiesel fuels have a low carbon to hydrogen content compared to fossil diesel. This gives biodiesel a 10% lower mass to energy content, but due to high density the biodiesel volumetric energy content drops about 5-6% compared to fossil diesel.
In other words, biodiesel has increased molecular weight compared to fossil diesel, reflected in the high distinction temperature (T90). Another important property exhibited by biodiesel fuel is good and excellent cetane number due to the straight chain esters compared to NO2 fossil diesel octane 93. Biodiesel fuels also especially renewable contain paraffinic hydrocarbons, dominated by odd carbon numbers [452-454]. Lastly comparing biodiesel viscosities with fossil diesel show higher values by a factor of 2 when compared to fossil diesel [455].
7.2. Kinematic Viscosity
Viscosity of a biodiesel fuel is a critical property. Viscosity plays a key role in the spray quality, mixture formation and ultimately influences the entire combustion process. In other words high kinematic viscosity interferes with the entire injection process thus leading to insufficient fuel atomization hence poor engine combustion and performance. The mean diameter of the atomized fuel droplets sprayed by the injector and their penetration increases as viscosity increases [456, 457]. Additionally high viscosity leads to rapid pressure rise in injection pumping system thus leading to advanced injection timing [458-462].
Among the leading problems associated with viscosity of fuel, include: Inefficient mixing of fuel which results into incomplete combustion [463]. Early injection due to the high line of pressure thus moving the start of combustion (SOC) closer to the top dead centre (TDC). This increases the maximum mean effective pressure leading to combustion chamber elevated temperature hence increased NOX emissions in biodiesel blends and fuels [464, 465]. As a property of fuel, viscosity is directly linked to the chemical structure of the fuel composition.
For example viscosity has been reported to increase as the carbon length increases and decreases with increased saturation (number of double bonds) of the biodiesel [214, 466]. Another important fuel property linked to the chemical structure via viscosity is the heat content (also known as the calorific value of a fuel) for both the feedstock and the biodiesel. For both the feedstock and biodiesel, the values increase together, in other words the heat content and viscosity increase or decrease together [214, 467].
Viscosity also is connected to the type of feedstock, for example, the viscosity of fats and greases is higher compared to vegetable oil sources. This is due to different saturation levels of the feedstock, which has been reported by a number of researchers such as [235, 468, 469]. Viscosity values for vegetable oil based feedstocks vary from between 27.2 mm2/s to 53.6 mm2/s compared to vegetable oil methyl esters at 3.6 mm2/s to 4.6 mm2/s. This phenomenon is due to the process of transesterification [470]. Nevertheless, despite this variation in viscosity due to feedstocks, biodiesel fuels have values relatively within specifications of prescribed standards.
7.3. Biodiesel Density
Density is described as the unit of mass per unit area. The density of a biodiesel causes the break-up of fuel injected into the engine cylinder. In other words, as the density of a fuel sample increases the mass of fuel-injected increases. However, regardless of the feedstock used to produce biodiesel oil all biodiesel are high in density compared to fossil diesel [215, 471-473]. The density of a fuel and its compressibility hold a very high influence in diesel engine fuel injection systems. Density thus influences the injected mass injection timing and the injection spray pattern.
These are critical parameters in biodiesel fuel and engine combustion behaviour [464, 474]. In other words, increasing the density increases the diameter of the droplets injected and considering the high inertia of heavier fuel droplets, this increases their penetration as denser fuel requires a shorter injection duration [456]. The speed at which the injected fuel spray penetrates across the combustion chamber determines air utilization and fuel /air ratio mixing rate [475].
Low-density fuel paired with low viscosity fuel when injected provides better and improved atomization and diffusion of the spray, which is an important factor in emission reduction and control. Atomization of the injected liquid fuel mass in the combustion chamber is important. Its importance is in the number of droplets, which are necessary for creating a large surface are for the liquid fuel to evaporate. This is governed by injection parameters and the air/fuel properties [475]. Density is also linked to the calorific value (heating content) of a fuel [214]. For example in literature reviewed density is shown to correlate to PM and NOX emissions and reported by many researchers in different experimental works such as [476-478].
The carbon chain and the level of saturation affect the density of fuel. In other words, these two factors can increase or lower density considerably depending on their interplaying factors. For example, biodiesel produced from fats and greases tend to be more saturated compared to vegetable oil. An increase in density for example from 860 kg/m3 for vegetable oil methyl ester (biodiesel) increases the viscosity from 3.59 mm2/s to 4.63 mm2/s [470] Hence, fats and greases produce high-density oil compared to vegetable based feedstocks. Nevertheless as alluded earlier this increase in density due to the difference in feedstock falls within acceptable standards a factor that has been reported in literature reviewed for example [479, 480].
7.4. Cetane Number
A cetane number in any given fuel is a primer indicator of the fuel ignition quality, which is the same as octane rating in SI fuel. The cetane number measures the knock tendency of a diesel fuel of biodiesel as a function of ignition delay. Although the cetane number is dimensionless, it is generally understood that ignition in compression ignition engines depends on self-ignition of the fuel.
Cetane number has been included as a fuel quality in biodiesel standard and placed at 47 as the minimum for neat biodiesel using ASTM standard. The cetane number also measures the readiness of a particular fuel to auto ignite when introduced into the combustion chamber for combustion. Hence the cetane number is a parameter that is directly proportional to ignition delay in IC engines [481].
Another general assumption made with cetane number is the conceptual generality of the octane scale for gasoline with petrodiesel cetane scale. For example high iso-octane as a primary reference fuel (PRF) has an octane rating of 100 compared to n-heptane at 0 [482]. On the other hand, the cetane scale the long straight chain hydrocarbon hexadecane (C16H34) is used as a PRF with an assigned CN of 100 compared to the highly branched hepta-methylnonane (C16H34) at 15. This confirms that branching and length of the carbon chain influence the CN [481]. In other words, increasing branching decreases the chain length hence the CN number also decreases or becomes smaller.
Ignition delay is a period between SOI and SOC. Ignition delay is heavily influenced by engine design parameters such as compression ratio, injection rate, injection time, inlet air temperature and fuel composition and fuel properties. As the cetane number increases, it directly decreases the ignition delay while increasing the phase combustion in diffusion-controlled combustion. Higher cetane numbers result into shorter ignition time. In other words, high cetane numbers reduce injection time, SOC and rapid pressure rise in diesel engine combustion ignition quality varies depending on the density, thus causing trouble during cold start and low load engine operating conditions. Other effects include long ignition delay, leading to increased and rapid pressure rise and high maximum combustion pressure factors, which are not desirable leading to rough engine operation.
In diesel engines, ignition knock is not desirable as it causes engine knock. Among influential effects of cetane number, cold flow and cold starting properties is the increase in smoke and engine noise emissions if found to be low. On the other hand, high cetane number causes SOC close to the injector nozzles hence overheating and nozzle premature failure. Secondly, it increases heat, and traps solid coated particles hence plugging the injector nozzles [472]. Because of this behaviour, a number of literature surveyed, limit the cetane number to below 65 [475]. Additionally a number of studies in literature surveyed on the effect of high cetane number report a correlation with reduced emissions [483-485]. For example smoke, UHC, NOX emissions reduce, with high CN. This has led to increased efforts to improve biodiesel fuel cetane numbers using additives or cetane improvers [463].
7.5. The Bulk Modulus of Compressibility
The bulk modulus of compressibility of a biodiesel explains and provides critical information on the number of spaces in biodiesel fuel molecules. The bulk modulus of compressibility also measures how much the biodiesel oil molecules can be compressed [486]. Although its measurement is difficult in liquids such as biodiesel, the measurement is obtained from the speed of sound and density of a biodiesel using the Newton-Laplace equation. This model utilizes estimates from a number of carbon and double bond of FAME in their chemical structures [487-489]. This is represented in Gibbs free energy (as shown in equations 10 and 11.
In other words, the bulk modulus of a biodiesel fuel is the reciprocal of its compressibility. This is the fractional change in volume per unit change in pressure P.
where
is the isentropic compressibility (Pa-1)
is the speed of sound (m.s-1)
Ρ is the density in kg.m-3
The bulk modulus of compressibility is a critical property in hydraulics such as biodiesel as it affects the hydraulic behaviour of fuels during injection hence its dilation [490]. The bulk modulus of compressibility is associated with the increase in NOX emissions in diesel and biodiesel variants. High NOx emissions produced by neat and blended biodiesel are linked to low compared to fossil diesel. This has been reported in a number of literature surveyed such as [491-495]. In Studies by [496], the authors established a linear relationship between isentropic compressibility of blends of biodiesel and NOx emission characteristics.
Therefore, the bulk modulus variation within the fuel composition and molecular structure is critical especially in biofuels, which are alternatives to fossil diesel. For example in literature surveyed, a number of experimental studies have been conducted in bulk modulus on biodiesel as alternative fuels and ULSD (Fisher Tropsch) [458, 497, 498]. This studies report advances in injection timing with use of biodiesel and ULSD fuels mainly due to the bulk modulus of compressibility and increased speed of sound. This leads to an earlier needle lift for in-line pump delivery systems [460, 499-502]. It is important to note that advanced injection timing in biodiesel and retarded timing in ULSD increases NOX as the blend ratio increases with the blend sample and vice versa [503, 504].
In the literature surveyed on the bulk modulus of compressibility there are a number of models used by different authors to estimate the speed of sound in biodiesel. Nevertheless, only two models are commonly used an Example of these authors who have conducted experimental work include [505-509].
7.6. The Calorific Value
The calorific value otherwise known as the energy content is energy per unit mass or the volume consumed during the process off combustion to give maximum energy output. The calorific value is also known as the heat of combustion and is numerically equal to the enthalpy of reaction [510]. The calorific value is measured using a bomb calorimeter under ASTM 2015. It is interesting to note that high-density fuels have greater energy content compared to low density fuels. Nevertheless they pack high energy content per unit mass in comparison [295]. In other words, when different fuels with different energy balances are used for performance testing and evaluation the same engine experiences different power outputs.
Biodiesel fuels as hydrocarbon compounds comprise n-saturated, unsaturated, branched cyclics. Vegetable oil, which form a bulk of biodiesel, contain three fatty acids called triglycerides. These fatty acids have a carbon chain length formed in a number of double bonds [511]. Most biodiesel oil contain 74.5 wt % to 78.4 wt%, hydrogen content of 10.6 wt% to 12.4 wt% and an oxygen content of 10.8 wt% to 12 wt % [463].
In other words, the elemental composition of fatty acids is important because it defines the energy content, through provision of weight percentage of carbon, hydrogen and oxygen components within a given sample of biodiesel. For example, the heating values of vegetable oil range from 24.29 MJ/kg to 41.20 MJ/kg. Vegetable source feedstocks have greater differences in heating values, but in surveyed literature, camelina has the highest HHV at 45.2MJ/kg in one study[414]. Others include corn and safflower with 43.1MJ/kg and 42.2MJ/kg respectively. It is also important to note in literature surveyed and data reported in literature there is confusion between this two terms LHV and HHV, which is confirmed in a number of studies such as [414, 448, 449].
Another observation found in the literature surveyed for commonly used biodiesel heating values indicates that they are few. Nevertheless, few researchers have managed to research and put some values for heating values together such as [512-514]. Hence, experimental determination of HHV of biodiesel fuels and other pure fatty acids is not exhaustive. Nevertheless a variety of correlations for predicting different HHV of fatty acids exist such as in (12 and 13 [513].
HHV, IV, SV and MW are high heating value, iodine value, saponification value and molecular weight of the fatty acids
Due to the high oxygen content biodiesel fuels have lower mass to energy, ratio values compared to fossil diesel fuels. Hence, it is generally accepted that biodiesel has 10% less mass energy content MJ/kg [462, 515]. The reduction in calorific value is mainly due to the presence of high oxygen content in the molecular structure of biodiesel fuels. These findings corroborate to a number of studies such as [463, 516, 517].
There are mainly two properties of biodiesel, which influence the calorific value, namely the saponification number and iodine values[518]. In other words, the decrease in saponification value reduces its molecular weight, which is similar to the effect of increased carbon and oxygen percentages in an oil sample. It is also important to note that in a given oil sample the calorific value is greatly affected by the iodine value [470]. The iodine value measures fuel properties of unsaturation.
The ASTM65751 nevertheless excludes it while the EN14214 specifies 120 mgI2/100g. On the other hand, the saponification value of biodiesel sample refers to a hydration reaction to break ester bonds using free hydroxide between fatty acid and glycerol of the triglycerides. The result forms free fatty acids and glycerol components soluble in aqueous solutions in (14.
![]() As observed from (12 the HHV can be determined using C, H, and O contents of the chemical structure as a function of percentage of these three components. However, (14 is a combination, of oxidative heat, values of C and H and the reduction heat of O with an assumption that the oxygen content effect on the fatty acid fuel has negative HHV. The determined values obtained by equations 12 and 13 and show the hydrogen content as the most decisive factor for unsaturated fatty acids [514]. Nevertheless, (13 shows HHV to be a functional component of the carbon percentage.
As observed from (12 the HHV can be determined using C, H, and O contents of the chemical structure as a function of percentage of these three components. However, (14 is a combination, of oxidative heat, values of C and H and the reduction heat of O with an assumption that the oxygen content effect on the fatty acid fuel has negative HHV. The determined values obtained by equations 12 and 13 and show the hydrogen content as the most decisive factor for unsaturated fatty acids [514]. Nevertheless, (13 shows HHV to be a functional component of the carbon percentage.
7.7. Oxidative Stability of Biodiesel
Biodiesel fuels degrade after storage for a long time due to oxidation. Hence, biodiesel stability refers to the ability of the biodiesel to resist degradation to form undesirable species and properties [519, 520]. This makes it possible for a biodiesel to resist physical and chemical changes caused by environmental factors. Nevertheless biodiesel fuels are none resistant to oxidation when exposed to air and moisture, this ultimately affects the biodiesel quality and storage. The existing time from initiation of oxidation to increased rate of oxidation is called induction period [521, 522]. During induction period, the concentration of ROOH is very low although this situation reverses as the reaction progresses.
In literature surveyed biodiesel fuel can degrade through a number of mechanisms [523] such as; Oxidation or autoxidation which occurs as a result of contact with ambient oxygen. The second mechanisms of oxidation is due to thermal oxidative decomposition due to exposure to excessive storage heat or direct light UV rays. The third mechanism comes from hydrolysis or accumulation of moisture or contact with water in storage tanks, fuel lines or moisture due to condensation. The fourth mechanism of biodiesel degradation is due to microbial or biodegradation contamination due to dust particles or water and moisture which contain bacteria or fungi into the storage tank or system. Metal contamination [524] and the Presence or absence of additives [525].
Besides these mechanisms biodiesel fuel itself is susceptible to oxidation and contamination through interaction with light and temperature. This is due to the presence of fatty acids, which interact with oxygen thus making biodiesel unstable. Besides nature and interaction, there are inherent chemical reactions such as alkenes, dienes and compounds of nitrogen, sulfur and oxygen, which hasten and play a dominant role on oxidation. The initial biodiesel products of oxidation are peroxides and hydro-peroxides, which when further degraded produce short chain hydrocarbons such as aldehydes, alcohols, ketones and low molecular compounds [520, 526, 527].
Among the visible physical changes to the naked eye is change in biodiesel physical colour, deposit formation that reduces biodiesel clarity and cleanliness [523, 528]. The degradation of biodiesel correlated to the process of transesterification, which either uses methanol or ethanol as each of them produces different esters. In other words during transesterification the fatty acid chain remains unchanged hence retaining the oxidation chemistry of the feedstock.
This is one of the leading explanation and cause of instability in biodiesel oil [519]. There are three types of oxidative stability identified with biodiesel fuels, which include oxidative stability [529, 530]. Storage stability which involves degradation due to interaction with light, air, metals, moisture and other storage related conditions [531, 532]. And Thermal stability which deals with oxidation at high temperature causing increased oil and fat [533, 534].
Nevertheless primary oxidation is further classified into three main reactions according to [519]. The first one is the initiation reaction where carbon free radicals are formed and produced [531, 535]. In other words, the diatomic oxygen present within the free radicals to form peroxy and further reaction leads to carbon free radical hydro-peroxide (ROOH). This is an extraction of the hydrogen atom from the carbon atom of the chain. The second reaction is the propagation reaction, which reacts the free carbon with atomic oxygen forming stable reaction products of two carbon free radicals, hence termination of the reaction. Vividly captured in equations 15 and 16 either through thermal dissociation of hydrogen peroxide.
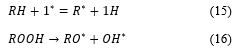 Alternatively, it takes a metal catalysed decomposition of the hydrogen peroxide as in equations 17, 18, 19 and 20.
Alternatively, it takes a metal catalysed decomposition of the hydrogen peroxide as in equations 17, 18, 19 and 20.
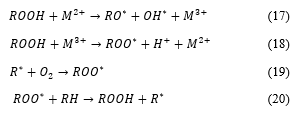 In the secondary oxidation reactions, the hydrogen-peroxide breaks down to form aldehydes of propanol, hexanals and heptanol while forming formic acid, aliphatic alcohol and formate esters. Additionally there is formation of short chain fatty acids, which lead to increased acid biodiesel value [536, 537].
In the secondary oxidation reactions, the hydrogen-peroxide breaks down to form aldehydes of propanol, hexanals and heptanol while forming formic acid, aliphatic alcohol and formate esters. Additionally there is formation of short chain fatty acids, which lead to increased acid biodiesel value [536, 537].
7.8. Biodiesel Lubricity Properties
The lubricity of biodiesel refers to the reduction of friction between solid surfaces relative to their motion [538]. Lubricity in biodiesel takes two main mechanisms namely; Hydrodynamic lubrication where a layer of a liquid such as blended biodiesel within the injection system prevents direct contact between opposing moving sides. The second is Boundary lubrication, which refers to compounds formulated to adhere to metallic surfaces while forming a thin protective layer that prevents wear.
These two forms of lubrication alternate with each other to provide lubrication especially boundary lubrication when hydrodynamic lubrication seizes to work or is removed from opposing surfaces. In other words, biodiesel oil should have good lubricity qualities, as this is critical in protecting moving parts in the modern injection systems. Modern day increased operational demands for injection systems is a critical factor and includes high and sustained pressure, injection rate shaping, multiple injection and engine injection cycles. Nevertheless, suffice to say that despite increased need for lubricity, natural lubricity from petroleum fuels has been decreasing with the advent of ULSD. This technology uses high level of hydrotreatment, which removes all heteroatom molecules of O, N and S which are key in improved lubricity[481, 539].
Generally, biodiesel is regarded with high and excellent lubricating properties a reason why blending with ULSD is recommended. This natural lubricity of biodiesel causes biodiesel to have no specification within ASTM and EN standards for B100. However blends B5 to B20 in ASTM7467 includes lubricity specifications[481]. Biodiesels excellent properties of lubricity traced to the esters group within its FAME molecules and other trace impure compounds. For example, free fatty acids and monoglycerides are effective in lubrication [540, 541]. In a number of studies reported in literature surveyed the authors note that the purification process by distillation decreases its lubricity as it removes impurities which are necessary to lubricity [539].
In literature surveyed, the effect of unsaturation on lubricity is inadequately covered and needs further study, as there is no comprehensive data. It is not clear from literature surveyed the role of unsaturation on lubricity and in the few available literatures, the results are mixed. For example, in a number of research work positive and negative effects of carbon-carbon double bonds is reported [215, 481, 542]. It is important to note that this impurities in biodiesel impact lubricity positively although they increase operational problems such as cold starting. Trying to reduce these impurities to improve cold flow properties of biodiesel has worsened the consequence of poor lubricity [414].
7.9. Cold Flow Biodiesel Properties (Flash Point, Pour Point and Cloud Point)
The cold flow properties of biodiesel indicate the ability of a biodiesel during cold weather and engine cold starting and is dependent on the long chain saturated factor [204]. Biodiesel fuels have similar and comparable physicochemical features as fossil diesel although their cold flow properties of the two fuels are dissimilar. All biodiesel fuels have very poor cold starting properties irrespective of the source of feedstock and blends without additives.
In biodiesel clod flow properties relate to the melting points of individual fuel components and their solubility in blends [543]. In other words, a high MP causes crystallization and precipitates once its blend goes beyond its solubility. Due to long chain saturated fatty acids, biodiesel fuel components exhibit higher MP compared to fossil diesel. However, when unsaturated fatty acid components are present the MP decreases. Table 15 shows CP for different feedstocks.
Table 15: Selected biodiesel feedstocks cloud points [544]
| Oil /Fat | Methyl ester composition (wt %) | Cloud point | ||||||
| C16:0 | C18:0 | C18:1 | C18:2 | C18:3 | Others | K | ℃ | |
| Beef tallow | 23.9 | 17.5 | 43.9 | 2.3 | 0.1 | 12.3 | 286 | 13 |
| Palm | 39.5 | 4.1 | 43.2 | 10.6 | 0.2 | 2.4 | 283 | 10 |
| Sunflower | 6.1 | 4.2 | 24 | 63.5 | 0.4 | 1.8 | 274 | 1 |
| Soybean | 10.7 | 3.2 | 25 | 53.3 | 5.4 | 2.5 | 272 | -1 |
| Linseed | 6.7 | 3.7 | 21.7 | 15.8 | 52.1 | 0 | 268 | -5 |
| Olive | 10.7 | 2.6 | 78.7 | 5.8 | 0.7 | 1.5 | 268 | -5 |
| Safflower | 6.4 | 2.2 | 13.9 | 76 | 0.2 | 1.3 | 267 | -6 |
| Rapeseed | 4.3 | 1.9 | 61.5 | 20.6 | 8.3 | 3.1 | 267 | -6 |
Nevertheless the flash point of biodiesel differs and refers to a safety measure of biodiesel storage as it’s a point at which a biodiesel fuel spontaneously becomes flammable [545]. On the other hand, the fire point of a hydrocarbon is a point at which a sample of fuel will continue to burn at its highest temperature and remains burning. The difference between the flash point and the fire point is 50 ℉ to 70 ℉. For example, fossil diesel has a flash point of 60 ℃ to 140℉ while the fire point is 93℃ to 200 ℉ ).
Table 16: Flash point and fire point of biodiesel and its blends
| Type of fuel | Flash point (℃) | Fire point (℃) |
| Diesel | 60 | 65 |
| Mahua oil | 286 | 295 |
| MME | 175 | 186 |
| Ethanol | 40 | 47 |
| Kerosene | 72 | 77 |
| MME 20 % ethanol | 50 | 55 |
| MME 10 % ethanol | 52 | 57 |
| MME 10 % ethanol 10% diesel | 54 | 59 |
| MME 20 % kerosene | 90 | 97 |
| MME 10 % kerosene | 95 | 101 |
The average flashpoint for biodiesel fuel is 150℃ compared to fossil diesel at 55℃ to 66℃ [546]. This difference is primarily due to the difference in their physicochemical properties. For example diesel fuel has low molecular weight molecules with branched compounds hence low flash point compared to biodiesel with trace of alcohol which reduces its flash point [450]. Due to the relationship between the biodiesel flashpoint and alcohol content, the flashpoint sets the limit of residual alcohol in a biodiesel or biofuel.
This means therefore that the flashpoint is an empirical measurement but not a fundamental physical biodiesel parameter and is inversely proportional to fuel volatility [547]. Higher ethanol blends are a fire hazard and as such should be discouraged as they reduce flashpoint and firepoints of biodiesel. Table 16 shows the Flash point and fire point of different biodiesel and their blends.
The pour point of biodiesel is defines as the lowest temperature point which a biodiesel fuel will still manage to flow before turning jelly and waxy [548]. The difference in CFPP value in biodiesel oil samples depend on the feedstock but relies more on the carbon chain length of the saturated oil fatty acids. For example palmitic acid (C16:0) in palm oil and (C12:0) and (C14:0) for coconut and Babassu respectively. In other words, carbon chains produce higher CFPP values [215, 414].
7.10. Acid Number Biodiesel Properties
The acid number of biodiesels is defined as the quantity of potassium hydroxide (KOH) which neutralizes fatty acids in a 1g sample. This is expressed in (form as follows in (21.
![]() The acid value is an important biodiesel property as it determines the amount of free fatty acids in a sample of fat; this is elaborated further in (8 [549]:
The acid value is an important biodiesel property as it determines the amount of free fatty acids in a sample of fat; this is elaborated further in (8 [549]:
S is the standard alkali used during titration of the sample in ml
B is the blank sample used during titration in ml
N is the normal standard alkali
W is the weight of the sample in grams
The biodiesel acid number also shows the sum total of all acid chemicals comprising the following: Phenols, Acids, Sugars, biodiesel oil extracts. Since biodiesel oil have high content of oxygen there is a high association with acidity linked to it [550]. This means the acid number measures the quantity of carboxylic acid groups in a chemical compound like fatty acid or as a mixture of compounds [551]. The acid number of biodiesel is contained in ASTM D 6751 using method ASTM D664 and EN14214 using method EN14104 [481]. Acid numbers quantify the acid values in a sample of biodiesel. Nevertheless Higher acid values cause a number of problems in injection systems by causing severe corrosion of internal component parts [551].
8. Conclusion and Future Recommendation
- Families and family generation are defined by the length of time, likewise the biodiesel families are defined by the length of time.
- Use of non-edible feedstocks is an answer to the challenges paused by edible oil feedstocks. The use of non-edible feedstocks in the production of biodiesel eliminates competition for food, reduction in deforestation rate and co-products waste.
- Besides chemical and physical factors, which affect biodiesel production tax and policy subsidy, completion with food industry, cost of feedstock and investment affect production of biodiesel.
- Although biodiesel is advantageous in many aspects, high cost of production, processing and raw materials (feedstock) which account for 80% make it expensive as an alternative.
- Viscosity plays a key role in the spray quality, mixture formation and ultimately influences the entire combustion process. In other words high kinematic viscosity interferes with the entire injection process thus leading to insufficient fuel atomization hence poor engine combustion and performance.
- The density of fuel is a prime role in the injected mass timing and the injection spray pattern. It is also important to remember that as the density of a fuel sample increases the mass of fuel-injected increases too.
- The cetane number in any given fuel is a primer indicator of the fuel ignition quality, which is the same as octane rating in SI fuel. The cetane number measures the knock tendency of a diesel fuel of biodiesel as a function of ignition delay.
- The elemental composition of fatty acids is important because it defines the energy content within a given sample of biodiesel. Through provision of weight percentage of carbon, hydrogen and oxygen components with a given sample of biodiesel it makes easy to determine the energy content.
- Biodiesel fuel is susceptible to oxidation and contamination through interaction with light and temperature due to the presence of fatty acids, which interact with oxygen. Visible physical changes to the naked eye to show this change in biodiesel include physical colour change of oil, deposit formation that reduces biodiesel clarity and cleanliness.
- Generally biodiesel is regarded with high and excellent lubricating properties a reason why blending with ULSD is recommended.
- Due to the relationship between the biodiesel flashpoint and alcohol content, the flashpoint sets the limit of residual alcohol in a biodiesel or biofuel. In other words although flashpoint is an empirical measurement but not a fundamental physical biodiesel parameter and is inversely proportional to fuel volatility.
- The feedstock type influences difference in the CFPP value in biodiesel oil samples although it relies more on the carbon chain length of the saturated oil fatty acids.
- The acid value has a significant impact on system component s life and performance. Higher acid values contribute to a number of problems in the injection systems sometimes resulting into severe corrosion of internal component parts for injection.
- E. Diesel, Diesel: Der Mensch, das Werk, das Schicksal. Mit 21 Bildern u. Wiedergaben im Text u. auf Kunstdrucktaf: Hanseatische Verlag-Anst., 1937.
- C. Chavanne, “Procédé de transformation d’huiles végétales en vue de leur utilisation comme carburants (Procedure for the transformation of vegetable oils in view of their use as fuels),” Belgian Patent BE, 422, 31, 1937.
- G. Chavanne, “Sur un mode d’utilisation possible de l’huile de palme à la fabrication d’un carburant lourd,” 1942.
- B. Kovarik, “Henry Ford, Charles F. Kettering and the fuel of the future,” Automotive History Review, 32, 7-27, 1998.
- J. Walton, “The fuel possibilities of vegetable oils,” Gas Oil Power, 33, 167-168, 1938.
- J. Bruwer, F. Hugo, and C. Hawkins, “Sunflower seed oil as an extender for diesel fuel in agricultural tractors,” 1980. NTIS (US Sales Only), PC A02/MF A01.
- S. Palash, M. Kalam, H. Masjuki, B. Masum, I. R. Fattah, and M. Mofijur, “Impacts of biodiesel combustion on NOx emissions and their reduction approaches,” Renewable and Sustainable Energy Reviews, 23, 473-490, 2013. https://doi.org/10.1016/j.rser.2013.03.003
- M. Mofijur, H. Masjuki, M. Kalam, M. Hazrat, A. Liaquat, M. Shahabuddin, et al., “Prospects of biodiesel from Jatropha in Malaysia,” Renewable and Sustainable Energy Reviews, 16, 5007-5020, 2012. https://doi.org/10.1016/j.rser.2013.03.003
- H. Ong, T. Mahlia, and H. Masjuki, “A review on energy scenario and sustainable energy in Malaysia,” Renewable and Sustainable Energy Reviews, 15, 639-647, 2011. https://doi.org/10.1016/j.rser.2010.09.043
- E. Cecrle, C. Depcik, A. Duncan, J. Guo, M. Mangus, E. Peltier, et al., “Investigation of the effects of biodiesel feedstock on the performance and emissions of a single-cylinder diesel engine,” Energy & Fuels, 26, 2331-2341, 2012. https://doi.org/10.1021/ef2017557
- A. Liaquat, M. Kalam, H. Masjuki, and M. Jayed, “Potential emissions reduction in road transport sector using biofuel in developing countries,” Atmospheric Environment, 44, 3869-3877, 2010. https://doi.org/10.1016/j.atmosenv.2010.07.003
- EIA, “Monthly biodiesel production report: january,” Energy Information Administration, Washington, DC, U.S2015.
- V. K. Kaimal and P. Vijayabalan, “A detailed study of combustion characteristics of a DI diesel engine using waste plastic oil and its blends,” Energy conversion and Management, 105, 951-956, 2015. https://doi.org/10.1016/j.enconman.2015.08.043
- I. Kalargaris, G. Tian, and S. Gu, “The utilisation of oils produced from plastic waste at different pyrolysis temperatures in a DI diesel engine,” Energy, 131, 179-185, 2017. https://doi.org/10.1016/j.energy.2017.05.024
- A. Sharma and S. Murugan, “Combustion, performance and emission characteristics of a DI diesel engine fuelled with non-petroleum fuel: a study on the role of fuel injection timing,” Journal of the Energy Institute, 88, 364-375, 2015. https://doi.org/10.1016/j.joei.2014.11.006
- A. K. Wamankar and S. Murugan, “Effect of injection timing on a DI diesel engine fuelled with a synthetic fuel blend,” Journal of the Energy Institute, 88, 406-413, 2015. https://doi.org/10.1016/j.joei.2014.11.003
- Department of Enviromental Affairs, “National Waste Management Strategy,” Department of Enviromental Affairs, Pretoria, South Africa2017.
- REN21, “Renewables Global Status Report: 2009 Update,” REN21 Secretariat,, Paris, France2009.
- Government of India, “National Policy on Biofuels,” New Delhi, India2009.
- K. M. Findlater and M. Kandlikar, “Land use and second-generation biofuel feedstocks: the unconsidered impacts of Jatropha biodiesel in Rajasthan, India,” Energy Policy, 39, 3404-3413, 2011. https://doi.org/10.1016/j.enpol.2011.03.037
- W. M. Achten, L. Verchot, Y. J. Franken, E. Mathijs, V. P. Singh, R. Aerts, et al., “Jatropha bio-diesel production and use,” Biomass and bioenergy, 32, 1063-1084, 2008. https://doi.org/10.1016/j.biombioe.2008.03.003
- P. Ariza-Montobbio and S. Lele, “Jatropha plantations for biodiesel in Tamil Nadu, India: Viability, livelihood trade-offs, and latent conflict,” Ecological Economics, 70, 189-195, 2010. https://doi.org/10.1016/j.ecolecon.2010.05.011
- B. Divakara, H. Upadhyaya, S. Wani, and C. L. Gowda, “Biology and genetic improvement of Jatropha curcas L.: a review,” Applied Energy, 87, 732-742, 2010. https://doi.org/10.1016/j.apenergy.2009.07.013
- E. Christoforou and P. A. Fokaides, Environmental Assessment of Solid Biofuels: Advances in Solid Biofuels, Springer, 2019. https://doi.org/10.1007/978-3-030-00862-8_6
- B. Amigun, J. K. Musango, and W. Stafford, “Biofuels and sustainability in Africa,” Renewable and sustainable energy reviews, 15, 1360-1372, 2011. https://doi.org/10.1016/j.rser.2010.10.015
- V. Buytaert, B. Muys, N. Devriendt, L. Pelkmans, J. Kretzschmar, and R. Samson, “Towards integrated sustainability assessment for energetic use of biomass: A state of the art evaluation of assessment tools,” Renewable and Sustainable Energy Reviews, 15, 3918-3933, 2011. https://doi.org/10.1016/j.rser.2011.07.036
- A. C. McBride, V. H. Dale, L. M. Baskaran, M. E. Downing, L. M. Eaton, R. A. Efroymson, et al., “Indicators to support environmental sustainability of bioenergy systems,” Ecological Indicators, 11, 1277-1289, 2011. https://doi.org/10.1016/j.ecolind.2011.01.010
- J. R. Seay and F. F. Badurdeen, “Current trends and directions in achieving sustainability in the biofuel and bioenergy supply chain,” Current opinion in chemical engineering, 6, 55-60, 2014. https://doi.org/10.1016/j.coche.2014.09.006
- V. H. Dale, R. A. Efroymson, K. L. Kline, M. H. Langholtz, P. N. Leiby, G. A. Oladosu, et al., “Indicators for assessing socioeconomic sustainability of bioenergy systems: a short list of practical measures,” Ecological Indicators, 26, 87-102, 2013. https://doi.org/10.1016/j.ecolind.2012.10.014
- V. H. Dale, R. A. Efroymson, K. L. Kline, and M. S. Davitt, “A framework for selecting indicators of bioenergy sustainability,” Biofuels, Bioproducts and Biorefining, vol. 9, 435-446, 2015. https://doi.org/10.1002/bbb.1562
- T. Buchholz, V. A. Luzadis, and T. A. Volk, “Sustainability criteria for bioenergy systems: results from an expert survey,” Journal of cleaner production, 17, S86-S98, 2009. https://doi.org/10.1016/j.jclepro.2009.04.015
- P. Fokaides and E. Christoforou, Life cycle sustainability assessment of biofuels: Handbook of biofuels production, Elsevier, 2016. https://doi.org/10.1016/B978-0-08-100455-5.00003-5
- C. Baskar, S. Baskar, and R. S. Dhillon, Biomass conversion: The interface of biotechnology, chemistry and materials science, Springer Science & Business Media, 2012.
- A. Azad, M. Rasul, M. Khan, S. C. Sharma, and M. Bhuiya, “Recent development of biodiesel combustion strategies and modelling for compression ignition engines,” Renewable and Sustainable Energy Reviews, 56, 1068-1086, 2016. https://doi.org/10.1016/j.rser.2015.12.024
- B. Singh, A. Guldhe, I. Rawat, and F. Bux, “Towards a sustainable approach for development of biodiesel from plant and microalgae,” Renewable and sustainable Energy reviews, 29, 216-245, 2014. https://doi.org/10.1016/j.rser.2013.08.067
- B. Singh, A. Guldhe, P. Singh, A. Singh, I. Rawat, and F. Bux, Sustainable production of biofuels from microalgae using a biorefinary approach: Applied environmental biotechnology: Present scenario and future trends, Springer, 2015. https://doi.org/10.1007/978-81-322-2123-4_8
- S. N. Naik, V. V. Goud, P. K. Rout, and A. K. Dalai, “Production of first and second generation biofuels: a comprehensive review,” Renewable and sustainable energy reviews, 14, 578-597, 2010. https://doi.org/10.1016/j.rser.2009.10.003
- E. Rostek, “Biofuels of first and second generation,” Journal of KONES, 23, 2016. doi: 10.5604/12314005.1217259
- W.-H. Leong, J.-W. Lim, M.-K. Lam, Y. Uemura, and Y.-C. Ho, “Third generation biofuels: A nutritional perspective in enhancing microbial lipid production,” Renewable and sustainable energy reviews, 91, 950-961, 2018. https://doi.org/10.1016/j.rser.2018.04.066
- Y. Chisti, “Biodiesel from microalgae,” Biotechnology advances, 25, 294-306, 2007. https://doi.org/10.1016/j.biotechadv.2007.02.001
- S. Behera, Singh, R., Arora, R., Sharma, N. K., Shukla, M., & Kumar, S,, “Scope of algae as third generation biofuels,” Frontiers in bioengineering and biotechnology, 2, 90, 2015. https://doi.org/10.3389/fbioe.2014.00090
- B. Zhao, Ma, J., Zhao, Q., Laurens, L., Jarvis, E., Chen, S., & Frear, C,, “Efficient anaerobic digestion of whole microalgae and lipid-extracted microalgae residues for methane energy production,” Bioresource technology, 161, 423-430, 2014. https://doi.org/10.1016/j.biortech.2014.03.079
- E. S. Salama, Kurade, M. B., Abou-Shanab, R. A., El-Dalatony, M. M., Yang, I. S., Min, B., & Jeon, B. H,, “Recent progress in microalgal biomass production coupled with wastewater treatment for biofuel generation,” Renewable and Sustainable Energy Reviews, 79, 1189-1211, 2017. https://doi.org/10.1016/j.rser.2017.05.091
- G. Markou, Wang, L., Ye, J., & Unc, A,, “Using agro-industrial wastes for the cultivation of microalgae and duckweeds: Contamination risks and biomass safety concerns, Biotechnology advances, 36(4), 1238-1254, 2018. https://doi.org/10.1016/j.biotechadv.2018.04.003
- W. A. Payne, Are biofuels antithetic to long-term sustainability of soil and water resources: Advances in agronomy, Academic Press, 2010. https://doi.org/10.1016/S0065-2113(10)05001-7
- N. K. Sharma, Tiwari, S. P., Tripathi, K., & Rai, A. K,, “Sustainability and cyanobacteria (blue-green algae): facts and challenges,” Journal of applied phycology, 23, 1059-1081, 2011. https://doi.org/10.1007/s10811-010-9626-3
- A. W. Sheppard, Gillespie, I., Hirsch, M., & Begley, C,, “Biosecurity and sustainability within the growing global bioeconomy,” Current Opinion in Environmental Sustainability, 3, 4-10, 2011. https://doi.org/10.1016/j.cosust.2010.12.011
- D. J. Patzelt, Hindersin, S., Elsayed, S., Boukis, N., Kerner, M., & Hanelt, D,, “Hydrothermal gasification of Acutodesmus obliquus for renewable energy production and nutrient recycling of microalgal mass cultures,” Journal of applied phycology, 27, 2239-2250, 2015. https://doi.org/10.1007/s10811-014-0496-y
- A. I. Barros, Gonçalves, A. L., Simões, M., & Pires, J. C,, “Harvesting techniques applied to microalgae: a review,” Renewable and Sustainable Energy Reviews, 41, 1489-1500, 2015. https://doi.org/10.1016/j.rser.2014.09.037
- D. J. Glass, “Pathways to obtain regulatory approvals for the use of genetically modified algae in biofuel or biobased chemical production,” Industrial Biotechnology, 11, 71-83, 2015. https://doi.org/10.1089/ind.2015.1503
- M. R. Tredici, “Photobiology of microalgae mass cultures: understanding the tools for the next green revolution,” Biofuels, 1(1),143-162, 2010. https://doi.org/10.4155/bfs.09.10
- G. Dragone, B. D. Fernandes, A. A. Vicente, and J. A. Teixeira, “Third generation biofuels from microalgae,” 2010. http://hdl.handle.net/1822/16807
- F. Alam, S. Mobin, and H. Chowdhury, “Third generation biofuel from Algae,” Procedia Engineering, 105, 763-768, 2015. https://doi.org/10.1016/j.proeng.2015.05.068
- W. H. Liew, M. H. Hassim, and D. K. Ng, “Review of evolution, technology and sustainability assessments of biofuel production,” Journal of Cleaner Production, 71, 11-29, 2014. https://doi.org/10.1016/j.jclepro.2014.01.006
- V. B. Agbor, N. Cicek, R. Sparling, A. Berlin, and D. B. Levin, “Biomass pretreatment: fundamentals toward application,” Biotechnology advances, 29, 675-685, 2011. https://doi.org/10.1016/j.biotechadv.2011.05.005
- P. Bajpai, Pretreatment of lignocellulosic biomass: Pretreatment of Lignocellulosic Biomass for Biofuel Production, Springer, 2016. https://doi.org/10.1007/978-981-10-0687-6_4
- K. Dutta, A. Daverey, and J.-G. Lin, “Evolution retrospective for alternative fuels: First to fourth generation,” Renewable energy, 69, 114-122, 2014. https://doi.org/10.1016/j.renene.2014.02.044
- M. F. Demirbas, “Current technologies for biomass conversion into chemicals and fuels,” Energy Sources, Part A: Recovery, Utilization, and Environmental Effects, 28, 1181-1188, 2006. https://doi.org/10.1080/00908310500434556
- P. McKendry, “Energy production from biomass (part 2): conversion technologies,” Bioresource technology, 83, 47-54, 2002. https://doi.org/10.1016/S0960-8524(01)00119-5
- P. Fokaides and P. Polycarpou, Exploitation of olive solid waste for energy purposes: Renewable energy, economies, emerging technologies and global practices. Nova Science Publishers, Inc, 2013.
- T. Brlek, N. Voća, T. Krička, J. Lević, Đ. Vukmirović, and R. Čolović, “Quality of pelleted olive cake for energy generation,” Agriculturae Conspectus Scientificus, 77, 31-35, 2012. https://hrcak.srce.hr/77902
- Y. Neubauer, Biomass gasification: Biomass Combustion Science, Technology and Engineering, Woodhead Publishing Series in Energy, 2013.
- F. Schüth, Hydrogen economics and its role in biorefining: Catalytic Hydrogenation for Biomass Valorization, Royal society of chemistry, 2014. doi: 10.1039/9781782620099-00001
- R. Reimert, F. Marschner, H. J. Renner, W. Boll, E. Supp, M. Brejc, et al., “Gas Production, 2. Processes,” Ullmann’s encyclopedia of industrial chemistry, 2000.
- A. Gómez-Barea and B. Leckner, “Modeling of biomass gasification in fluidized bed,” Progress in Energy and Combustion Science, 36, 444-509, 2010. https://doi.org/10.1016/j.pecs.2009.12.002
- A. E. Abdelaziz, Leite, G. B., & Hallenbeck, P. C., “Addressing the challenges for sustainable production of algal biofuels: II. Harvesting and conversion to biofuels,” Environmental technology, 34, 1807-1836, 2013. https://doi.org/10.1080/09593330.2013.831487
- X. Ji and X. Long, “A review of the ecological and socioeconomic effects of biofuel and energy policy recommendations,” Renewable and Sustainable Energy Reviews, 61, 41-52, 2016. https://doi.org/10.1016/j.rser.2016.03.026
- J. P. Maity, J. Bundschuh, C.-Y. Chen, and P. Bhattacharya, “Microalgae for third generation biofuel production, mitigation of greenhouse gas emissions and wastewater treatment: Present and future perspectives–A mini review,” Energy, 78, 104-113, 2014. https://doi.org/10.1016/j.energy.2014.04.003
- USDOE, “National Algal Biofuels Technology Review,” Bioenergy Technologies Office, Washington DC, USA2016.
- S. A. Scott, M. P. Davey, J. S. Dennis, I. Horst, C. J. Howe, D. J. Lea-Smith, et al., “Biodiesel from algae: challenges and prospects,” Current opinion in biotechnology, 21, 277-286, 2010. https://doi.org/10.1016/j.copbio.2010.03.005
- I. Christian and A. John, “Feasibility of Second and Third Generation Biofuel in General Aviation: A Research Report and Analysis,” McNair Scholars Research Journal, 1, 4, 2014. https://commons.erau.edu/mcnair/vol1/iss1/4
- G. B. Leite, A. E. Abdelaziz, and P. C. Hallenbeck, “Algal biofuels: challenges and opportunities,” Bioresource technology, 145, 134-141, 2013. https://doi.org/10.1016/j.biortech.2013.02.007
- T. W. Hertel, W. E. Tyner, and D. K. Birur, “The global impacts of biofuel mandates,” The Energy Journal, 31(1-4), 75-100, 2010. doi: 10.5547/ISSN0195-6574-EJ-Vol31-No1-4
- M. Yumurtaci and A. Kecebas, “Renewable energy and its university level education in Turkey,” Energy Education Science and Technology Part B-Social and Educational Studies, 3, 143-152, 2011.
- K. F. Yee, K. T. Tan, A. Z. Abdullah, and K. T. Lee, “Life cycle assessment of palm biodiesel: revealing facts and benefits for sustainability,” Applied Energy, 86, S189-S196, 2009. https://doi.org/10.1016/j.apenergy.2009.04.014
- A. Demirbas, “Progress and recent trends in biodiesel fuels,” Energy conversion and management, 50, 14-34, 2009. https://doi.org/10.1016/j.enconman.2008.09.001
- Y. Sahin, “Environmental impacts of biofuels,” Energy Education Science and Technology Part A-Energy Science and Research, 26, 129-142, 2011. doi:10.3155/1047-3289.61.3.285
- A. Demirbas, “Political, economic and environmental impacts of biofuels: A review,” Applied energy, 86, S108-S117, 2009. https://doi.org/10.1016/j.apenergy.2009.04.036
- M. F. Demirbas, “Biofuels from algae for sustainable development,” Applied energy, vol. 88, 3473-3480, 2011. https://doi.org/10.1016/j.apenergy.2011.01.059
- J. P. Hewett, Wolfe, A. K., Bergmann, R. A., Stelling, S. C., & Davis, K. L,, “Human health and environmental risks posed by synthetic biology R&D for energy applications: a literature analysis,” Applied Biosafety, 21, 177-184, 2016. https://doi.org/10.1177/1535676016672377
- M. Y. Menetrez, “An overview of algae biofuel production and potential environmental impact,” Environmental science & technology, 46, 7073-7085, 2012. https://doi.org/10.1021/es300917r
- S. Genitsaris, Kormas, K. A., & Moustaka-Gouni, M,, “Airborne algae and cyanobacteria: occurrence and related health effects,” Frontiers in Bioscience, 3, 772-787, 2011.
- O. Wright, Stan, G. B., & Ellis, T,, “Building-in biosafety for synthetic biology,” Microbiology, 159, 1221-1235, 2013. https://doi.org/10.1099/mic.0.066308-0
- G. N. Mandel, “Gaps, inexperience, inconsistencies, and overlaps: Crisis in the regulation of genetically modified plants and animals,” William & Mary Law Review, 45, 2167, 2003.
- G. E. Marchant, & Wallach, W, Governing the governance of emerging technologies: Innovative governance models for emerging technologies, Edward Elgar Publishing, 2013. https://doi.org/10.4337/9781782545644.00013
- A. A. Snow, & Smith, V. H,, “Genetically engineered algae for biofuels: a key role for ecologists,” Bioscience, 62, 765-768, 2012. https://doi.org/10.1525/bio.2012.62.8.9
- W. J. Henley, Litaker, R. W., Novoveská, L., Duke, C. S., Quemada, H. D., & Sayre, R. T, “Initial risk assessment of genetically modified (GM) microalgae for commodity-scale biofuel cultivation,” Algal Research, 2, 66-77, 2013. https://doi.org/10.1016/j.algal.2012.11.001
- J. B. Tucker, & Zilinskas, R. A,, “The promise and perils of synthetic biology,” The New Atlantis, 12, 25-45, 2006. https://www.jstor.org/stable/43152238
- A. Bhutkar, “Synthetic biology: navigating the challenges ahead,” Journal of Biolaw & Business, vol. 8, 19-29, 2005.
- T. Kuiken, Dana, G., Oye, K., & Rejeski, D, “Shaping ecological risk research for synthetic biology,” Journal of Environmental Studies and Sciences, 4, 191-199, 2014. https://doi.org/10.1007/s13412-014-0171-2
- A. Raybould, “The bucket and the searchlight: formulating and testing risk hypotheses about the weediness and invasiveness potential of transgenic crops,” Environmental biosafety research, 9, 123-133, 2010. https://doi.org/10.1051/ebr/2011101
- S. Lu, Li, L., & Zhou, G,, “Genetic modification of wood quality for second-generation biofuel production,” GM crops, 1, 230-236, 2010.
- K. Bhattarai, Brummer, E. C., & Monteros, M. J,, “Alfalfa as a bioenergy crop; Bioenergy FeedstocksBreeding and Genetics, Taylor and Francis, 2013. https://doi.org/10.1002/9781118609477.ch10
- J. M. Jeschke, Keesing, F., & Ostfeld, R. S, “Novel organisms: comparing invasive species, GMOs, and emerging pathogens,” Ambio, 45, 541-548, 2013. https://doi.org/10.1007/s13280-013-0387-5
- F. J. Areal, Riesgo, L., & Rodriguez-Cerezo, E,, ” Economic and agronomic impact of commercialized GM crops: a meta-analysis,” The Journal of Agricultural Science, 151, 7-33, 2013. doi:10.1017/S0021859612000111
- S. J. Smyth, W. A. Kerr, and P. W. Phillips, “Global economic, environmental and health benefits from GM crop adoption,” Global Food Security, 7, 24-29, 2015. https://doi.org/10.1016/j.gfs.2015.10.002
- M. D. Edgerton, “Increasing crop productivity to meet global needs for feed, food, and fuel,” Plant physiology, 149, 7-13, 2009. https://doi.org/10.1104/pp.108.130195
- R. Muñoz and C. Gonzalez-Fernandez, Microalgae-based biofuels and bioproducts: from feedstock cultivation to end-products, Woodhead Publishing, 2017.
- P. Tandon and Q. Jin, “Microalgae culture enhancement through key microbial approaches,” Renewable and Sustainable Energy Reviews, 80, 1089-1099, 2017. https://doi.org/10.1016/j.rser.2017.05.260
- B. M. Wolf, D. M. Niedzwiedzki, N. C. M. Magdaong, R. Roth, U. Goodenough, and R. E. Blankenship, “Characterization of a newly isolated freshwater Eustigmatophyte alga capable of utilizing far-red light as its sole light source,” Photosynthesis research, 135, 177-189, 2018. doi: 10.1007/s11120-017-0401-z
- D. E. T. Cervantes, A. L. Martínez, M. C. Hernández, and A. L. G. de Cortázar, “Using indicators as a tool to evaluate municipal solid waste management: A critical review,” Waste management, 80, 51-63, 2018. https://doi.org/10.1016/j.wasman.2018.08.046
- B. R. Alzamora and R. T. d. V. Barros, “Review of municipal waste management charging methods in different countries,” Waste Management, 115, 47-55, 2020. https://doi.org/10.1016/j.wasman.2020.07.020
- S. Chinnasamy, Rao, P. H., Bhaskar, S., Rengasamy, R., & Singh, M, Algae a novel biomass feedstock for biofuels: Microbial biotechnology Energy and environment, 224-239, 2012.
- B. Sajjadi, Chen, W. Y., Raman, A. A. A., & Ibrahim, S, “Microalgae lipid and biomass for biofuel production: A comprehensive review on lipid enhancement strategies and their effects on fatty acid composition,” Renewable and Sustainable Energy Reviews, 97, 200-232, 2018. https://doi.org/10.1016/j.rser.2018.07.050
- A. C. Guedes, Amaro, H. M., & Malcata, F. X, “Microalgae as sources of carotenoids,” Marine drugs, 9, 625-644, 2011. https://doi.org/10.3390/md9040625
- B. Abdullah, Muhammad, S. A. F. A. S., Shokravi, Z., Ismail, S., Kassim, K. A., Mahmood, A. N., & Aziz, M. M. A,, “Fourth generation biofuel: A review on risks and mitigation strategies,” Renewable and sustainable energy reviews, vol. 107, 37-50, 2019. https://doi.org/10.1016/j.rser.2019.02.018
- A. Srivastava, & Torres-Vargas, C. E,, “Genetically Modified Crops: A Long Way to Go: Environmental Issues Surrounding Human Overpopulation, IGI Global, 2017. doi: 10.4018/978-1-5225-1683-5.ch006
- I. Atadashi, M. Aroua, and A. A. Aziz, “High quality biodiesel and its diesel engine application: a review,” Renewable and sustainable energy reviews, 14, 1999-2008, 2010. https://doi.org/10.1016/j.rser.2010.03.020
- L. Lin, Z. Cunshan, S. Vittayapadung, S. Xiangqian, and D. Mingdong, “Opportunities and challenges for biodiesel fuel,” Applied energy, vol. 88, 1020-1031, 2011.
- J. C. Bart, Palmeri, N., & Cavallaro, S, Biodiesel science and technology: from soil to oil, Woodhead Publishing Elsevier, 2010.
- H. Hao, Liu, Z., Zhao, F., Ren, J., Chang, S., Rong, K., & Du, J, “Biofuel for vehicle use in China: Current status, future potential and policy implications.,” Renewable and Sustainable Energy Reviews, vol. 82, 645-653, 2018. https://doi.org/10.1016/j.rser.2017.09.045
- Y. Sani, W. Daud, and A. Abdul Raman, Biodesel feedstock and production technologies: successes, challenges and prospects 4, Intech, 2013. http://dx.org/10.5772/52790
- S. P. Souza, Seabra, J. E., & Nogueira, L. A. H, “Feedstocks for biodiesel production: Brazilian and global perspectives,” Biofuels, 9, 455-478, 2018. https://doi.org/10.1080/17597269.2017.1278931
- A. E. Atabani, El-Sheekh, M. M., Kumar, G., & Shobana, S, Edible and nonedible biodiesel feedstocks: microalgae and future of biodiesel: Clean Energy for Sustainable Development, Academic Press-Elsevier, 2017. https://doi.org/10.1016/B978-0-12-805423-9.00017-X
- Y. C. Sharma, Singh, B., & Korstad, J. (2009). H, “High yield and conversion of biodiesel from a nonedible feedstock (Pongamia pinnata),” Journal of agricultural and food chemistry, 58, 242-247, 2009. https://doi.org/10.1021/jf903227e
- I. Ozkurt, “Qualifying of safflower and algae for energy,” Energy Education Science and Technology Part A-Energy Science and Research, 23, 145-151, 2009.
- G. El Diwani, Attia, N. K., & Hawash, S. I, “Development and evaluation of biodiesel fuel and by-products from jatropha oil,” International Journal of Environmental Science & Technology, 6, 219-224, 2009.
- B. Sanjay, “Yellow oleander (Thevetia peruviana) seed oil biodiesel as an alternative and renewable fuel for diesel engines: a review,” International Journal of ChemTech Research, 7, 2823-2840, 2015. http://sphinxsai.com/2015/ch_vol7_no6…
- A. E. Atabani, Silitonga, A. S., Badruddin, I. A., Mahlia, T. M. I., Masjuki, H. H., & Mekhilef, S,, “A comprehensive review on biodiesel as an alternative energy resource and its characteristics,” Renewable and sustainable energy reviews, 16, 2070-2093, 2012. https://doi.org/10.1016/j.rser.2012.01.003
- M. Tabatabaei, K. Karimi, I. Horvath, and R. Kumar, “Recent trends in biodiesel production, ,” Biofuel Research Journal, 7, 258-267, 2015. doi: 10.18331/BRJ2015.2.3.4
- L. F. Razon, “Alternative crops for biodiesel feedstock. CAB Reviews,” Perspectives in agriculture, veterinary science, nutrition and natural resources, vol. 4, 1-15, 2009.
- A. L. Ahmad, Yasin, N. M., Derek, C. J. C., & Lim, J. K, “Microalgae as a sustainable energy source for biodiesel production: a review,” Renewable and Sustainable Energy Reviews, 15, 584-593, 2011. https://doi.org/10.1016/j.rser.2010.09.018
- USDA, “United States Department of Agriculture,” Washington, D.C., United States2017.
- REN21, “Renewables 2015 – global status report,” REN21, Paris, France2015.
- USDA, “Biofuel annual: European union,” United States Department of Agriculture, Office of Global Analysis, Foreign Agricultural Service, The Hague, Netherlands2014.
- USDA, “Biofuels annual: argentina. Buenos Aires,” United States Department of Agriculture, Office of Global Analysis, Foreign Agricultural Service Washington, D.C., United States2014.
- USDA, “Biofuels annual: Indonesia. Jakarta,” United States Department of Agriculture, Office of Global Analysis, Foreign Agricultural Service, Washington, D.C., United States2014.
- USDA, “Biofuels annual: Thailand. Bangkok,” United States Department of Agriculture, Office of Global Analysis, Foreign Agricultural Service;, Washington, D.C., United States3/24/2009 2009.
- A. De Oliveira, Brazilian Agroenergy Plan: 2006-2011, Embrapa Publishing House, 2011.
- FAO. Production, crops Online. Available: http://faostat3.fao.org/download/Q/QC/E
- Market Intel. China Uses One-Third of World’s Soybeans Online. Available: https://www.fb.org/market-intel/china-uses-one-third-of-worlds-soybeans
- J. Hilliard and T. Daynard, “Measurement of protein and oil in grains and soybeans with reflected near–infrared light,” Canadian Institute of Food Science and Technology Journal, vol. 9, 11-14, 1976.
- USDA. Oilseeds: world markets and trade Online.
- Y.-S. Song, J. Frías, C. Martinez-Villaluenga, C. Vidal-Valdeverde, and E. G. de Mejia, “Immunoreactivity reduction of soybean meal by fermentation, effect on amino acid composition and antigenicity of commercial soy products,” Food Chemistry, vol. 108, 571-581, 2008. https://doi.org/10.1016/j.foodchem.2007.11.013
- N. Antolović, V. Kožul, M. Antolović, and J. Bolotin, “Effects of partial replacement of fish meal by soybean meal on growth of juvenile saddled bream (Sparidae),” Turkish Journal of Fisheries and Aquatic Sciences, 12, 247-252, 2012. doi : 10.4194/1303-2712-v12_2_08
- S. Cools, W. Van den Broeck, L. Vanhaecke, A. Heyerick, P. Bossaert, M. Hostens, et al., “Feeding soybean meal increases the blood level of isoflavones and reduces the steroidogenic capacity in bovine corpora lutea, without affecting peripheral progesterone concentrations,” Animal reproduction science, 144, 9-89, 2014. https://doi.org/10.1016/j.anireprosci.2013.12.008
- M. Hernández, F. Martínez, M. Jover, and B. G. García, “Effects of partial replacement of fish meal by soybean meal in sharpsnout seabream (Diplodus puntazzo) diet,” Aquaculture, vol. 263, 159-167, 2007. https://doi.org/10.1016/j.aquaculture.2006.07.040
- M. P. Hojilla-Evangelista, “Adhesion properties of plywood glue containing soybean meal as an extender,” Journal of the American Oil Chemists’ Society, 87, 1047-1052, 2010. doi 10.1007/s11746-010-1586-x
- FAO. Production, crops processed Online.
- A. L. Mourad and A. Walter, “The energy balance of soybean biodiesel in Brazil: a case study,” Biofuels, Bioproducts and Biorefining, vol. 5, 185-197, 2011. https://doi.org/10.1002/bbb.278
- J. Fargione, J. Hill, D. Tilman, S. Polasky, and P. Hawthorne, “Land clearing and the biofuel carbon debt,” Science, 319, 1235-1238, 2008. doi: 10.1126/science.1152747
- M. H. Rocha, R. S. Capaz, E. E. S. Lora, L. A. H. Nogueira, M. M. V. Leme, M. L. G. Renó, et al., “Life cycle assessment (LCA) for biofuels in Brazilian conditions: a meta-analysis,” Renewable and Sustainable Energy Reviews, 37, 435-459, 2014. https://doi.org/10.1016/j.rser.2014.05.036
- D. S. Kim, M. Hanifzadeh, and A. Kumar, “Trend of biodiesel feedstock and its impact on biodiesel emission characteristics,” Environmental Progress & Sustainable Energy, 37, 7-19, 2018. https://doi.org/10.1002/ep.12800
- C. O’Shea, P. Mc Alpine, P. Solan, T. Curran, P. Varley, A. Walsh, et al., “The effect of protease and xylanase enzymes on growth performance, nutrient digestibility, and manure odour in grower–finisher pigs,” Animal Feed Science and Technology, vol. 189, 88-97, 2014. https://doi.org/10.1016/j.anifeedsci.2013.11.012
- L. Zhu, J. Wang, X. Ding, S. Bai, Q. Zeng, Z. Su, et al., “Effects of dietary rapeseed meal on laying performance, egg quality, apparent metabolic energy, and nutrient digestibility in laying hens,” Livestock science, 214, 265-271, 2018. https://doi.org/10.1016/j.livsci.2018.06.007
- M. Abduh, R. Manurung, and H. Heeres, “Techno-economic analysis for small scale production of rubber seed oil and biodiesel in Palangkaraya, Indonesia,” Journal of Clean Energy Technologies, 5, 268-273, 2017.
- S. Ghosal. Castor oil prices spike 23% in global markets Online. Available:https://economictimes.indiatimes.com/markets/commodities/news/castor-oil-prices-spike-23-in-global market/articleshow/ 69089709
- P. Halder, N. Paul, and M. Beg, “Prospect of Pongamia pinnata (Karanja) in Bangladesh: a sustainable source of liquid fuel,” Journal of Renewable Energy, 2014, 2014. https://doi.org/10.1155/2014/647324
- F. Dalemans, B. Muys, and M. Maertens, “A framework for profitability evaluation of agroforestry based biofuel value chains: An application to pongamia in India,” GCB Bioenergy, 2019. https://doi.org/10.1111/gcbb.12605
- J. P. Wanasundara, T. C. McIntosh, S. P. Perera, T. S. Withana-Gamage, and P. Mitra, “Canola/rapeseed protein-functionality and nutrition,” OCL, 23, D407, 2016. https://doi.org/10.1051/ocl/2016028
- J. Bergmann, D. Tupinambá, O. Costa, J. Almeida, C. Barreto, and B. Quirino, “Biodiesel production in Brazil and alternative biomass feedstocks,” Renewable and Sustainable Energy Reviews, 21, 411-420, 2013. https://doi.org/10.1016/j.rser.2012.12.058
- R. He, X. Ju, J. Yuan, L. Wang, A. T. Girgih, and R. E. Aluko, “Antioxidant activities of rapeseed peptides produced by solid state fermentation,” Food Research International, 49, 432-438, 2012. https://doi.org/10.1016/j.foodres.2012.08.023
- A. Marjanović-Jeromela, R. Marinković, A. Mijić, M. Jankulovska, Z. Zdunić, and N. Nagl, “Oil yield stability of winter rapeseed (Brassica napus L.) genotypes,” Agriculturae Conspectus Scientificus, 73, 217-220, 2008. https://hrcak.srce.hr/31240
- I. R. Fattah, H. Masjuki, A. Liaquat, R. Ramli, M. Kalam, and V. Riazuddin, “Impact of various biodiesel fuels obtained from edible and non-edible oils on engine exhaust gas and noise emissions,” Renewable and Sustainable Energy Reviews, 18, 552-567, 2013. https://doi.org/10.1016/j.rser.2012.10.036
- T. Searchinger, Heimlich, R., Houghton, R. A., Dong, F., Elobeid, A., Fabiosa, J., … & Yu, T. H, “Use of US croplands for biofuels increases greenhouse gases through emissions from land-use change,” Science, 319, 1238-1240, 2008. doi: 10.1126/science.1151861
- S. P. De Souza, S. Pacca, M. T. De Ávila, and J. L. B. Borges, “Greenhouse gas emissions and energy balance of palm oil biofuel,” Renewable Energy, 35, 2552-2561, 2010. https://doi.org/10.1016/j.renene.2010.03.028
- M. Umikalsom, A. Ariff, H. Zulkifli, C. Tong, M. Hassan, and M. Karim, “The treatment of oil palm empty fruit bunch fibre for subsequent use as substrate for cellulase production by Chaetomium globosum Kunze,” Bioresource Technology, 62, 1-9, 1997. https://doi.org/10.1016/S0960-8524(97)00132-6
- P. Adhikari, X.-M. Zhu, A. Gautam, J.-A. Shin, J.-N. Hu, J.-H. Lee, et al., “Scaled-up production of zero-trans margarine fat using pine nut oil and palm stearin,” Food chemistry, 119, 1332-1338, 2010. https://doi.org/10.1016/j.foodchem.2009.09.009
- C. Lesage and L. Feintrenie, Are sustainable pathways possible for oil palm development in Latin America: Land governance in an interconnected world, Washington, USA, 2018. https://agritrop.cirad.fr/587928/
- A. Simeh and T. Ahmad, The case study on the Malaysian palm oil: Regional Workshop on commodity export diversification and poverty reduction in South and South-East Asia, Bangkok, UNCTAD, 2001.
- K. Tan, K. Lee, A. Mohamed, and S. Bhatia, “Palm oil: addressing issues and towards sustainable development,” Renewable and sustainable energy reviews, 13, 420-427, 2009. https://doi.org/10.1016/j.rser.2007.10.001
- S. Sumathi, S. Chai, and A. Mohamed, “Utilization of oil palm as a source of renewable energy in Malaysia,” Renewable and sustainable energy reviews, 12, 2404-2421, 2008. https://doi.org/10.1016/j.rser.2007.06.006
- F. Arrieta, F. Teixeira, E. Yáñez, E. Lora, and E. Castillo, “Cogeneration potential in the Columbian palm oil industry: Three case studies,” Biomass and Bioenergy, 31, 503-511, 2007. https://doi.org/10.1016/j.biombioe.2007.01.016
- A. D. Padula, M. S. Santos, L. Ferreira, and D. Borenstein, “The emergence of the biodiesel industry in Brazil: current figures and future prospects,” Energy policy, 44, 395-405, 2012. https://doi.org/10.1016/j.enpol.2012.02.003
- A. da Silva César, M. O. Batalha, and A. L. M. S. Zopelari, “Oil palm biodiesel: Brazil’s main challenges,” Energy, 60, 485-491, 2013. https://doi.org/10.1016/j.energy.2013.08.014
- A. N. Payl and M. Mashud, “Experimental investigation on fuel properties of biodiesel prepared from cottonseed oil,” Proceedings in 2017 AIP Conference , 020018, 2017. https://doi.org/10.1063/1.4984647
- J.-M. Lacape, G. Gawrysiak, T.-V. Cao, C. Viot, D. Llewellyn, S. Liu, et al., “Mapping QTLs for traits related to phenology, morphology and yield components in an inter-specific Gossypium hirsutum× G. barbadense cotton RIL population,” Field Crops Research, 144, 256-267, 2013. https://doi.org/10.1016/j.fcr.2013.01.001
- D. Sinha and S. Murugavelh, “Biodiesel production from waste cotton seed oil using low cost catalyst: Engine performance and emission characteristics,” Perspectives in science, 8, 237-240, 2016. https://doi.org/10.1016/j.pisc.2016.04.038
- G. M. P. de Faria, M. da Silva Oliveira, L. P. de Carvalho, and C. D. Cruz, “Gains from selection for oil content in cotton,” Industrial crops and products, 51, 370-375, 2013. https://doi.org/10.1016/j.indcrop.013.09.005
- S. F. Vaughn, N. A. Deppe, M. A. Berhow, and R. L. Evangelista, “Lesquerella press cake as an organic fertilizer for greenhouse tomatoes,” Industrial crops and products, 32, 164-168, 2010. https://doi.org/10.1016/j.indcrop.2010.04.008
- M. H. Li and E. H. Robinson, “Use of cottonseed meal in aquatic animal diets: a review,” North American Journal of Aquaculture, vol. 68, 14-22, 2006.
- S. Adeel, S. Ali, I. A. Bhatti, and F. Zsila, “Dyeing of cotton fabric using pomegranate (Punica granatum) aqueous extract,” Asian Journal of Chemistry, 21, 3493, 2009.
- N. Reddy and Y. Yang, “Properties and potential applications of natural cellulose fibers from the bark of cotton stalks,” Bioresource technology, 100, 3563-3569, 2009. https://doi.org/10.1016/j.biortech.2009.02.047
- F. D. Gunstone, J. L. Harwood, and A. J. Dijkstra, The lipid handbook with CD-ROM, CRC press, 2007.
- I. Balalić, M. Zorić, G. Branković, S. Terzić, and J. Crnobarac, “Interpretation of hybrid× sowing date interaction for oil content and oil yield in sunflower,” Field Crops Research, 137, 70-77, 2012. https://doi.org/10.1016/j.fcr.2012.08.005
- V. D. Zheljazkov, B. A. Vick, B. S. Baldwin, N. Buehring, C. Coker, T. Astatkie, et al., “Oil productivity and composition of sunflower as a function of hybrid and planting date,” Industrial Crops and Products, 33, 537-543, 2011. https://doi.org/10.1016/j.indcrop.2010.11.004
- G. O. India. Post Harvest Profile of Sunflower, Directorate of Marketing and Inspection (Nagpur Branch) Online. Available: http://Agmarknet.Nic.In/Sunflower_Profile.Pdf
- N. Ramulu, K. Murthy, H. Jayadeva, M. Venkatesha, and H. Ravi Kumar, “Seed yield and nutrients uptake of sunflower (Helianthus annuusL.) as influenced by different levels of nutrients under irrigated condition of eastern dry zone of Karnataka, India,” Plant Arch, 11, 1061-1066, 2011.
- G. Ragaglini, F. Triana, R. Villani, and E. Bonari, “Can sunflower provide biofuel for inland demand? An integrated assessment of sustainability at regional scale,” Energy, 36, 2111-2118, 2011. https://doi.org/10.1016/j.energy.2010.03.009
- M. Karamać, A. Kosińska, I. Estrella, T. Hernández, and M. Duenas, “Antioxidant activity of phenolic compounds identified in sunflower seeds,” European Food Research and Technology, vol. 235, 221-230, 2012. https://doi.org/10.1007/s00217-012-1751-6
- Y. Ulusoy, R. Arslan, and C. Kaplan, “Emission characteristics of sunflower oil methyl ester,” Energy Sources, Part A, 31, 906-910, 2009. https://doi.org/10.1080/15567030802087528
- C. d. Castro and R. d. C. LEITE, “Main aspects of sunflower production in Brazil,” Embrapa Soja-Artigo em periódico indexado (ALICE), 2018. https://doi.org/10.1051/ocl/2017056
- C. E. Feoli and J. Ingaramo, South America perspectives on sunflower production and processing: Sunflower, Elsevier, 2015. https://doi.org/10.1016/B978-1-893997-94-3.50023-4
- P. Debaeke, P. Casadebaig, F. Flenet, and N. Langlade, “Sunflower crop and climate change: vulnerability, adaptation, and mitigation potential from case-studies in Europe,” Ocl, 24, D102, 2017. doi : 10.1051/ocl/2016052
- M. A. Khan, M. Akmal, and M. Afzal, “Fertilizer N-and P-rates response on sunflower inter-cropping with Mungbean in North-West, Pakistan,” Basic Res. J. Agri. Rev, 3, 146-160, 2014.
- J. M. Cerveró, J. Coca, and S. Luque, “Production of biodiesel from vegetable oils,” Grasas y aceites, 59, 76-83, 2008. https://doi.org/10.3989/gya.2008.v59.i1.494
- G. A. Gbogouri, K. Brou, G. A. M. Beugre, D. Gnakri, and M. Linder, “Assessment of the thermo-oxidation of three cucurbit seed oils by differential scanning calorimetry,” Innovative Romanian Food Biotechnology, 12, 32, 2013.
- A. Saydut, A. B. Kafadar, Y. Tonbul, C. Kaya, F. Aydin, and C. Hamamci, “Comparison of the biodiesel quality produced from refined sunflower (Helianthus annuus L) oil and waste cooking oil,” Energy Exploration & Exploitation, 28, 499-512, 2010. https://doi.org/10.1260/0144-5987.28.6.499
- B. B. Marvey, “Sunflower-based feedstocks in nonfood applications: perspectives from olefin metathesis,” International journal of molecular sciences, 9, 1393-1406, 2008. https://doi.org/10.3390/ijms9081393
- J. Dyer, L. Stringer, and A. Dougill, “Jatropha curcas: Sowing local seeds of success in Malaw: In response to Achten et al.(2010),” Journal of Arid Environments, 79, 107-110, 2012. https://doi.org/10.1016/j.jaridenv.2011.12.004
- C. M. dos Santos, V. Verissimo, H. C. de Lins Wanderley Filho, V. M. Ferreira, P. G. da Silva Cavalcante, E. V. Rolim, et al., “Seasonal variations of photosynthesis, gas exchange, quantum efficiency of photosystem II and biochemical responses of Jatropha curcas L. grown in semi-humid and semi-arid areas subject to water stress,” Industrial Crops and Products, 41, 203-213, 2013. https://doi.org/10.1016/j.indcrop.2012.04.003
- A. Thanapimmetha, A. Luadsongkram, B. Titapiwatanakun, and P. Srinophakun, “Value added waste of Jatropha curcas residue: optimization of protease production in solid state fermentation by Taguchi DOE methodology,” Industrial Crops and Products, 37, 1-5, 2012. https://doi.org/10.1016/j.indcrop.2011.11.003
- R. Chandra, V. Vijay, P. Subbarao, and T. Khura, “Production of methane from anaerobic digestion of jatropha and pongamia oil cakes,” Applied Energy, 93, 148-159, 2012. https://doi.org/10.1016/j.apenergy.2010.10.049
- P. Kant and S. Wu, “The extraordinary collapse of Jatropha as a global biofuel,” ACS Publications, 2011.
- B. Singh, K. Singh, G. R. Rao, J. Chikara, D. Kumar, D. Mishra, et al., “Agro-technology of Jatropha curcas for diverse environmental conditions in India,” Biomass and bioenergy, 48, 191-202, 2013. https://doi.org/10.1016/j.biombioe.2012.11.025
- C. Everson, M. Mengistu, and M. B. Gush, “A field assessment of the agronomic performance and water use of Jatropha curcas in South Africa,” Biomass and Bioenergy, 59, 59-69, 2013. https://doi.org/10.1016/j.biombioe.2012.03.013
- M. P. Dorado, “Raw materials to produce low-cost biodiesel,” Biofuels refining and performance, 107-147, 2008.
- B. R. Moser, “Biodiesel production, properties, and feedstocks: Vitro Cellular & Developmental Biology-Plant, 45, 2009. https://doi.org/10.1007/978-1-4419-7145-6_15
- S. Pinzi, Garcia, I. L., Lopez-Gimenez, F. J., Luque de Castro, M. D., Dorado, G., & Dorado, M. P,, “The ideal vegetable oil-based biodiesel composition: a review of social, economical and technical implications,” Energy & Fuels, 23, 2325-2341, 2009. https://doi.org/10.1021/ef801098a
- M. Balat, “Potential alternatives to edible oils for biodiesel production–A review of current work,” Energy conversion and management, 52, 1479-1492, 2011. https://doi.org/10.1016/j.enconman.2010.10.011
- L. Azócar, Heipieper, H. J., & Navia, R,, “Biotechnological processes for biodiesel production using alternative oils.,” Applied microbiology and biotechnology, 88, 621-636, 2010. https://doi.org/10.1007/s00253-010-2804-z
- A. Ramadhas, S. Jayaraj, and C. Muraleedharan, “Use of vegetable oils as IC engine fuels—a review,” Renewable energy, 29, 727-742, 2004. https://doi.org/10.1016/j.renene.2003.09.008
- A. Atabani, A. Silitonga, H. Ong, T. Mahlia, H. Masjuki, I. A. Badruddin, et al., “Non-edible vegetable oils: a critical evaluation of oil extraction, fatty acid compositions, biodiesel production, characteristics, engine performance and emissions production,” Renewable and sustainable energy reviews, 18, 211-245, 2013. https://doi.org/10.1016/j.rser.2012.10.013
- A. Demirbas, “Biodiesel from corn germ oil catalytic and non-catalytic supercritical methanol transesterification,” Energy Sources, Part A: Recovery, Utilization, and Environmental Effects, 38, 1890-1897, 2016. https://doi.org/10.1080/15567036.2015.1004388
- M. Haile, “Integrated volarization of spent coffee grounds to biofuels,” Biofuel Research Journal, 1, 65-69, 2014.
- I. B. Banković-Ilić, Stojković, I. J., Stamenković, O. S., Veljkovic, V. B., & Hung, Y. T,, “Waste animal fats as feedstocks for biodiesel production. ,” Renewable and sustainable energy reviews, vol. 32, 238-254, 2014. https://doi.org/10.1016/j.rser.2014.01.038
- T. Maneerung, Kawi, S., Dai, Y., & Wang, C. H, , “Sustainable biodiesel production via transesterification of waste cooking oil by using CaO catalysts prepared from chicken manure,” Energy Conversion and Management, 123, 487-497, 2016. https://doi.org/10.1016/j.enconman.2016.06.071
- Z. Yaakob, M. Mohammad, M. Alherbawi, Z. Alam, and K. Sopian, “Overview of the production of biodiesel from waste cooking oil,” Renewable and sustainable energy reviews, 18, 184-193, 2013. https://doi.org/10.1016/j.rser.2012.10.016
- Y. Chen, Xiao, B., Chang, J., Fu, Y., Lv, P., & Wang, X,, “Synthesis of biodiesel from waste cooking oil using immobilized lipase in fixed bed reactor,” Energy conversion and management, 50, 668-673, 2009. https://doi.org/10.1016/j.enconman.2008.10.011
- S. M. Hingu, P. R. Gogate, and V. K. Rathod, “Synthesis of biodiesel from waste cooking oil using sonochemical reactors,” Ultrasonics sonochemistry, 17, 827-832, 2010. https://doi.org/10.1016/j.ultsonch.2010.02.010
- C. G. Lopresto, Naccarato, S., Albo, L., De Paola, M. G., Chakraborty, S., Curcio, S., & Calabrò, V,, “Enzymatic transesterification of waste vegetable oil to produce biodiesel,” Ecotoxicology and environmental safety, 121, 2015. https://doi.org/10.1016/j.ecoenv.2015.03.028
- M. Corral Bobadilla, Lostado Lorza, R., Escribano García, R., Somovilla Gómez, F., & Vergara González, E,, “An improvement in biodiesel production from waste cooking oil by applying thought multi-response surface methodology using desirability functions,” Energies, 10, 130, 2017. https://doi.org/10.3390/en10010130
- A. Demirbas, “Relationships derived from physical properties of vegetable oil and biodiesel fuels,” Fuel, 87, 1743-1748, 2008. https://doi.org/10.1016/j.fuel.2007.08.007
- G. Knothe, “Dependence of biodiesel fuel properties on the structure of fatty acid alkyl esters,” Fuel processing technology, vol. 86, 1059-1070, 2005. https://doi.org/10.1016/j.fuproc.2004.11.002
- P. Poltronieri, Tobacco seed oil for biofuels: Biotransformation of Agricultural Waste and By-Products, Elsevier, 2016. https://doi.org/10.1016/B978-0-12-803622-8.00006-9
- M. M. Gui, K. Lee, and S. Bhatia, “Feasibility of edible oil vs. non-edible oil vs. waste edible oil as biodiesel feedstock,” Energy, 33, 1646-1653, 2008. https://doi.org/10.1016/j.energy.2008.06.002
- B. Sanjay, “Non-conventional seed oils as potential feedstocks for future biodiesel industries: a brief review,” Research Journal of Chemical Sciences, 2013. Available online at: www.isca.in
- W. Du, & Liu, D. H, Biodiesel from conventional feedstocks: Biotechnology in China III: Biofuels and Bioenergy, Springer, 2012. https://doi.org/10.1007/10_2011_127
- M. G. Kulkarni, & Dalai, A. K,, “Waste cooking oil an economical source for biodiesel: a review,” Industrial & engineering chemistry research, 45, 2901-2913, 2006. https://doi.org/10.1021/ie0510526
- H. Zhang, Xu, Z., Zhou, D., & Cao, J,, “Waste cooking oil-to-energy under incomplete information: Identifying policy options through an evolutionary game.,” Applied energy, 185, 547-555, 2017. https://doi.org/10.1016/j.apenergy.2016.10.133
- H. Vaghari, Jafarizadeh-Malmiri, H., Mohammadlou, M., Berenjian, A., Anarjan, N., Jafari, N., & Nasiri, S, “Application of magnetic nanoparticles in smart enzyme immobilization,” Biotechnology letters, 38, 223-233, 2016. https://doi.org/10.1007/s10529-015-1977-z
- Y. Jiang, & Zhang, Y,, “Supply chain optimization of biodiesel produced from waste cooking oil,” Transportation Research Procedia, 12, 938-949, 2016.
- M. A. Gonzalez-Salazar, Venturini, M., Poganietz, W. R., Finkenrath, M., Kirsten, T., Acevedo, H., & Spina, P. R,, “Development of a technology roadmap for bioenergy exploitation including biofuels, waste-to-energy and power generation & CHP,” Applied energy, 180, 338-352, 2016. https://doi.org/10.1016/j.apenergy.2016.07.120
- M. N. Hussain, Al Samad, T., & Janajreh, I, “Economic feasibility of biodiesel production from waste cooking oil in the UAE,” Sustainable cities and Society, 26, 217-226, 2016. https://doi.org/10.1016/j.scs.2016.06.010
- S. Cho, Kim, J., Park, H. C., & Heo, E, “Incentives for waste cooking oil collection in South Korea: a contingent valuation approach,” Resources, Conservation and Recycling, 99, 63-71, 2015. https://doi.org/10.1016/j.resconrec.2015.04.003
- N. Escobar, Ribal, J., Clemente, G., Rodrigo, A., Pascual, A., & Sanjuán, N, “Uncertainty analysis in the financial assessment of an integrated management system for restaurant and catering waste in Spain,” The International Journal of Life Cycle Assessment, 20, 1491-1510, 2015. https://doi.org/10.1007/s11367-015-0962-z
- S. Liang, Liu, Z., Xu, M., & Zhang, T, “Waste oil derived biofuels in China bring brightness for global GHG mitigation,” Bioresource technology, 131, 139-145, 2013. https://doi.org/10.1016/j.biortech.2012.12.008
- H. Zhang, Zhou, Q., Chang, F., Pan, H., Liu, X. F., Li, H., … & Yang, S,, “Production and fuel properties of biodiesel from Firmiana platanifolia Lf as a potential non-food oil source,” Industrial Crops and Products, 76, 768-771, 2015. https://doi.org/10.1016/j.indcrop.2015.08.002
- J. Rodrigues, Oliveira, V., Lopes, P., & Dias-Ferreira, C,, “Door-to-door collection of food and kitchen waste in city centers under the framework of multimunicipal waste management systems in Portugal: the case study of Aveiro,” Waste and biomass valorization, vol. 6, pp. 647-656, 2015. https://doi.org/10.1007/s12649-015-9366-3
- C. Sheinbaum-Pardo, Calderón-Irazoque, A., & Ramírez-Suárez, M,, “Potential of biodiesel from waste cooking oil in Mexico,” Biomass and bioenergy, 56, 230-238, 2013. https://doi.org/10.1016/j.biombioe.2013.05.008
- Wikipedia. Biodiesel Online. Available: http://en.wikipedia.org/wiki/BiodieselS.
- C. Öner, & Altun, Ş,, “Biodiesel production from inedible animal tallow and an experimental investigation of its use as alternative fuel in a direct injection diesel engine,” Applied energy, 86, 2114-2120, 2009. https://doi.org/10.1016/j.apenergy.2009.01.005
- M. Gürü, B. D. Artukoğlu, A. Keskin, and A. Koca, “Biodiesel production from waste animal fat and improvement of its characteristics by synthesized nickel and magnesium additive,” Energy Conversion and Management, 50, 498-502, 2009. https://doi.org/10.1016/j.enconman.2008.11.001
- J. M. Encinar, Sánchez, N., Martínez, G., & García, L, “Study of biodiesel production from animal fats with high free fatty acid content,” Bioresource Technology, 102, 10907-10914, 2011. https://doi.org/10.1016/j.biortech.2011.09.068
- A. C. J. Vivian Feddern, Marina Celant De Prá,, J. I. d. S. F. Paulo Giovanni de Abreu, and M. S. a. A. C. Martha Mayumi Higarashi, “Animal Fat Wastes for Biodiesel Production: Biodiesel: feedstocks and processing technologies, BoD–Books on Demand, 2011.
- S. K. Dash, & Lingfa, P, “A review on production of biodiesel using catalyzed transesterification,” 020100, July 2017. https://doi.org/10.1063/1.4990253
- J. V. Gerpen. Animal Fats for Biodiesel Production Online. Available:https://farm-energy.extension.org/animal-fats-for-biodiesel-production
- J. Janaun and N. Ellis, “Perspectives on biodiesel as a sustainable fuel,” Renewable and Sustainable Energy Reviews, 14, 1312-1320, 2010. https://doi.org/10.1016/j.rser.2009.12.011
- M. E. Tat, Van Gerpen, J. H., & Wang, P. S, “Fuel property effects on injection timing, ignition timing and oxides of nitrogen emissions from biodiesel-fueled engines,” in ASAE Annual Meeting, Minneapolis, Minnesota, USA, 1, 2004.
- A. Kerihuel, M. S. Kumar, J. Bellettre, and M. Tazerout, “Ethanol animal fat emulsions as a diesel engine fuel–Part 1: Formulations and influential parameters,” Fuel, 85, 2640-2645, 2006. https://doi.org/10.1016/j.fuel.2006.05.002
- K. Satyanarayana, A. Mariano, and J. Vargas, “A review on microalgae, a versatile source for sustainable energy and materials,” International Journal of energy research, 35, 291-311, 2011. https://doi.org/10.1002/er.1695
- T. J. Lundquist, I. C. Woertz, N. Quinn, and J. R. Benemann, “A realistic technology and engineering assessment of algae biofuel production,” Energy Biosciences Institute, 1, 2010.
- A. Demirbas and M. F. Demirbas, “Importance of algae oil as a source of biodiesel,” Energy conversion and management, 52, 163-170, 2011. https://doi.org/10.1016/j.enconman.2010.06.055
- L. Brennan and P. Owende, “Biofuels from microalgae—a review of technologies for production, processing, and extractions of biofuels and co-products,” Renewable and sustainable energy reviews, 14, 557-577, 2010. https://doi.org/10.1016/j.rser.2009.10.009
- T. M. Mata, A. A. Martins, and N. S. Caetano, “Microalgae for biodiesel production and other applications: a review,” Renewable and sustainable energy reviews, 14, 217-232, 2010. https://doi.org/10.1016/j.rser.2009.07.020
- G. Petkov, A. Ivanova, I. Iliev, and I. Vaseva, “A critical look at the microalgae biodiesel,” European Journal of Lipid Science and Technology, 114, 103-111, 2012. https://doi.org/10.1002/ejlt.201100234
- H. Xu, X. Miao, and Q. Wu, “High quality biodiesel production from a microalga Chlorella protothecoides by heterotrophic growth in fermenters,” Journal of biotechnology, 126, 499-507, 2006. https://doi.org/10.1016/j.jbiotec.2006.05.002
- A. S. Carlsson, J. B. Van Beilen, R. Möller, and D. Clayton, “Micro-and macro-algae: utility for industrial applications,” CPL Press, 2007.
- D. Vandamme, S. C. V. Pontes, K. Goiris, I. Foubert, L. J. J. Pinoy, and K. Muylaert, “Evaluation of electro-coagulation–flocculation for harvesting marine and freshwater microalgae,” Biotechnology and bioengineering, 108, 2320-2329, 2011. https://doi.org/10.1002/bit.23199
- H. C. Greenwell, L. Laurens, R. Shields, R. Lovitt, and K. Flynn, “Placing microalgae on the biofuels priority list: a review of the technological challenges,” Journal of the royal society interface, 7, 703-726, 2009. https://doi.org/10.1098/rsif.2009.0322
- K. Sikes, M. Van Walwijk, and R. McGill, “Algae as a Feedstock for Biofuels: An Assessment of the state of technology and Opportunities,” Final Report, 1-23, 2010.
- Y. Chisti, “Biodiesel from microalgae beats bioethanol,” Trends in biotechnology, 26, 126-131, 2008. https://doi.org/10.1016/j.tibtech.2007.12.002
- P. Gerbens-Leenes, G. de Vries, and L. Xu, “The water footprint of biofuels from microalgae,” Bioenergy and Water, 191, 2013.
- G. Brownbridge, P. Azadi, A. Smallbone, A. Bhave, B. Taylor, and M. Kraft, “The future viability of algae-derived biodiesel under economic and technical uncertainties,” Bioresource technology, 151, 166-173, 2014. https://doi.org/10.1016/j.biortech.2013.10.062
- R. Kothari, A. Pandey, S. Ahmad, A. Kumar, V. V. Pathak, and V. Tyagi, “Microalgal cultivation for value-added products: a critical enviro-economical assessment,” 3 Biotech, 7, 243, 2017. https://doi.org/10.1007/s13205-017-0812-8
- Y.-T. Hung, L. K. Wang, and N. K. Shammas, Handbook of environment and waste management: air and water pollution control 1, World Scientific, 2012.
- A. Demirbas, “New biorenewable fuels from vegetable oils,” Energy sources, Part A: recovery, utilization, and environmental effects, 32, 628-636, 2010. https://doi.org/10.1080/15567030903058832
- M. F. Demirbas, M. Balat, and H. Balat, “Biowastes-to-biofuels,” Energy Conversion and Management, 52, 1815-1828, 2011. https://doi.org/10.1016/j.enconman.2010.10.041
- L. S. Oliveira and A. S. Franca, “From solid biowastes to liquid biofuels,” Agriculture Issues and Policies Series, vol. 265, 2009.
- T. Michaels, “The 2007 IWSA directory of waste-to-energy plants,” Integrated Waste Services Association, 12, 32-45, 2010.
- Lux Research Inc. The Cleantech Report. Waste to energy Online. Available: http://www.luxresearchinc.com/pdf/08CTR_tech_profile
- M.-A. Perea-Moreno, E. Samerón-Manzano, and A.-J. Perea-Moreno, “Biomass as renewable energy: Worldwide research trends,” Sustainability, 11, 863, 2019. https://doi.org/10.3390/su11030863
- L. Visser, R. Hoefnagels, and M. Junginger, “The Potential Contribution of Imported Biomass to Renewable Energy Targets in the EU–the Trade-off between Ambitious Greenhouse Gas Emission Reduction Targets and Cost Thresholds,” Energies, 13, 1761, 2020. https://doi.org/10.3390/en13071761
- I. Dunmade, Application of Lifecycle Concepts in the Conversion of Biomass to Value-Added Commodities: Valorization of Biomass to Value-Added Commodities, Springer, 2020. https://doi.org/10.1007/978-3-030-38032-8_25
- R. Kataki, N. J. Bordoloi, R. Saikia, D. Sut, R. Narzari, L. Gogoi, et al., Waste Valorization to Fuel and Chemicals Through Pyrolysis: Technology, Feedstock, Products, and Economic Analysis: Waste to Wealth, Springer, 2018. https://doi.org/10.1007/978-981-10-7431-8_21
- S.-Y. No, Application of Liquid Biofuels to Internal Combustion Engines, Springer Nature, 2020.
- A. H. Demirbas and I. Demirbas, “Importance of rural bioenergy for developing countries,” Energy Conversion and Management, 48, 2386-2398, 2007. https://doi.org/10.1016/j.enconman.2007.03.005
- A. Demirbas, “Comparison of thermochemical conversion processes of biomass to hydrogen-rich gas mixtures,” Energy Sources, Part A: Recovery, Utilization, and Environmental Effects, 38, 2971-2976, 2016.
- K. R. Jegannathan, E.-S. Chan, and P. Ravindra, “Harnessing biofuels: a global Renaissance in energy production?,” Renewable and Sustainable Energy Reviews, 13, 2163-2168, 2009. https://doi.org/10.1016/j.rser.2009.01.012
- A. P. Darzins, Philip Edye, Les “Current status and potential for algal biofuels production,” J A report to IEA Bioenergy Task, 39, 2010.
- M. Balat, “An overview of biofuels and policies in the European Union,” J Energy Sources, Part B, 2, 167-181, 2007.
- T. L. C. Walker, Chris Purton, Saul,, “Algal Transgenics In The Genomic Era 1,” J Journal of Phycology, 41, 1077-1093, 2005.
- M. Vohra, J. Manwar, R. Manmode, S. Padgilwar, and S. Patil, “Bioethanol production: feedstock and current technologies,” Journal of Environmental Chemical Engineering, vol. 2, 573-584, 2014. https://doi.org/10.1016/j.jece.2013.10.013
- S. L. Stattman, A. Gupta, L. Partzsch, and P. Oosterveer, “Toward sustainable biofuels in the European Union? Lessons from a decade of hybrid biofuel governance,” Sustainability, 10, 4111, 2018. https://doi.org/10.3390/su10114111
- A. Hernández-García, S. B. Velásquez-Orta, E. Novelo, I. Yáñez-Noguez, I. Monje-Ramírez, and M. T. O. Ledesma, “Wastewater-leachate treatment by microalgae: Biomass, carbohydrate and lipid production,” Ecotoxicology and Environmental safety, 174, 435-444, 2019. https://doi.org/10.1016/j.ecoenv.2019.02.052
- R. Samiee-Zafarghandi, J. Karimi-Sabet, M. A. Abdoli, and A. Karbassi, “Increasing microalgal carbohydrate content for hydrothermal gasification purposes,” Renewable Energy, 116, 710-719, 2018. https://doi.org/10.1016/j.renene.2017.10.020
- M. Hanifzadeh, E. C. Garcia, and S. Viamajala, “Production of lipid and carbohydrate from microalgae without compromising biomass productivities: Role of Ca and Mg,” Renewable Energy, 127, 989-997, 2018. https://doi.org/10.1016/j.renene.2018.05.012
- K. T. Chen, Cheng, C. H., Wu, Y. H., Lu, W. C., Lin, Y. H., & Lee, H. T, , “Continuous lipid extraction of microalgae using high-pressure carbon dioxide,” Bioresource technology, 146, 23-26, 2013. https://doi.org/10.1016/j.biortech.2013.07.017
- R. A. Bush and K. M. Hall, “Process for the production of ethanol from algae,” Google Patents, 2009.
- H. Sun, K.Hu, H. Lou, and X. Zheng, “Biodiesel Production from Transesterification of Rapeseed Oil Using KF/Eu 2O 3 as a Catalyst,” Energy & Fuels, 22, 2756-2760, 2008.
- S. Nasreen, M. Nafees, L. A. Qureshi, M. S. Asad, A. Sadiq, and S. D. Ali, “Review of Catalytic Transesterification Methods for Biodiesel Production,” Biofuels: State of Development, 93, 2018.
- S. Mohapatra, P. Das, D. Swain, S. Satapathy, and S. R. Sahu, “A Review on Rejuvenated Techniques in Biodiesel Production from Vegetable Oils,” International Journal of Current Engineering and Technology, vol. 6, 100-111, 2016.
- H. T. HAJY and K. Tahvildari, “Efficient Synthesis of biodiesel from waste cooking oil catalysed by Al2O3 impregnated with NaOH,” 2015.
- M. Yadav and Y. C. Sharma, “Transesterification of used vegetable oil using BaAl2O4 spinel as heterogeneous base catalyst,” Energy Conversion and Management, 198, 111795, 2019. https://doi.org/10.1016/j.enconman.2019.111795
- N. Al-Jammal, Z. Al-Hamamre, and M. Alnaief, “Manufacturing of zeolite based catalyst from zeolite tuft for biodiesel production from waste sunflower oil,” Renewable Energy, 93, 449-459, 2016. https://doi.org/10.1016/j.renene.2016.03.018
- M. R. Anuar and A. Z. Abdullah, “Challenges in biodiesel industry with regards to feedstock, environmental, social and sustainability issues: a critical review,” Renewable and Sustainable Energy Reviews, 58, 208-223, 2016. https://doi.org/10.1016/j.rser.2015.12.296
- S. Lim and L. K. Teong, “Recent trends, opportunities and challenges of biodiesel in Malaysia: an overview,” Renewable and Sustainable Energy Reviews, 14, 938-954, 2010. https://doi.org/10.1016/j.rser.2009.10.027
- A. Z. Abdullah, B. Salamatinia, H. Mootabadi, and S. Bhatia, “Current status and policies on biodiesel industry in Malaysia as the world’s leading producer of palm oil,” Energy Policy, 37, 5440-5448, 2009. https://doi.org/10.1016/j.enpol.2009.08.012
- S. Mekhilef, S. Siga, and R. Saidur, “A review on palm oil biodiesel as a source of renewable fuel,” Renewable and Sustainable Energy Reviews, 15, 1937-1949, 2011. https://doi.org/10.1016/j.rser.2010.12.012
- Y. Ariani and S. Yuliar, Translating Biofuel, Discounting Farmers: The Search for Alternative Energy in Indonesia: Actor-Network Theory and Technology Innovation: Advancements and New Concepts, IGI Global, 2011. doi: 10.4018/978-1-60960-197-3.ch005
- M. Lister, Unmasking the Invisible Giant: Energy Efficiency in the Politics of Climate and Energy: Energy Security in the Era of Climate Change, Springer, 2012, https://doi.org/10.1057/9780230355361_3
- A. Chanthawong and S. Dhakal, “Liquid biofuels development in southeast asian countries: an analysis of market, policies and challenges,” Waste and biomass valorization, 7, 157-173, 2016. https://doi.org/10.1007/s12649-015-9433-9
- J. C. Kurnia, S. V. Jangam, S. Akhtar, A. P. Sasmito, and A. S. Mujumdar, “Advances in biofuel production from oil palm and palm oil processing wastes: a review,” Biofuel Research Journal, 3, 332-346, 2016. doi:10.18331/BRJ2016.3.1.3
- O. M. Ali, Mamat, R., Rasul, M. G., & Najafi, G, Potential of Biodiesel as Fuel for Diesel Engine: Clean Energy for Sustainable Development, Academic Press, 2017. https://doi.org/10.1016/B978-0-12-805423-9.00018-1
- D. M. Marinković, M. V. Stanković, A. V. Veličković, J. M. Avramović, M.R. Miladinović, O. O. Stamenković, et al., “Calcium oxide as a promising heterogeneous catalyst for biodiesel production: Current state and perspectives,” Renewable and Sustainable Energy Reviews, 56, 1387-1408, 2016. https://doi.org/10.1016/j.rser.2015.12.007
- H. Bateni, A. Saraeian, and C. Able, “A comprehensive review on biodiesel purification and upgrading,” Biofuel Research Journal, 4, 668-690, 2017.
- R. Brunet, Carrasco, D., Muñoz, E., Guillén-Gosálbez, G., Katakis, I., & Jiménez, L, “Economic and environmental evaluation of microalgae biodiesel production using process simulation tools,” in 22nd European Symposium on Computer Aided Process Engineering, University College, London, UK, 547-551, 2012. https://doi.org/10.1016/B978-0-444-59519-5.50110-6
- K. Colombo, Ender, L., & Barros, A. A. C, “The study of biodiesel production using CaO as a heterogeneous catalytic reaction,” Egyptian Journal of Petroleum, 26, 341-349, 2017. https://doi.org/10.1016/j.ejpe.2016.05.006
- A. Gaurav, Ng, F. T., & Rempel, G. L, “A new green process for biodiesel production from waste oils via catalytic distillation using a solid acid catalyst–Modeling, economic and environmental analysis,” Green Energy & Environment, 1, 62-74, 2016. https://doi.org/10.1016/j.gee.2016.05.003
- S. Karmee, Patria, R., & Lin, C, “Techno-economic evaluation of biodiesel production from waste cooking oil—a case study of Hong Kong,” International journal of molecular sciences, 16, 4362-4371, 2015. https://doi.org/10.3390/ijms16034362
- J. Price, Nordblad, M., Martel, H. H., Chrabas, B., Wang, H., Nielsen, P. M., & Woodley, J. M, “Scale-up of industrial biodiesel production to 40 m3 using a liquid lipase formulation,” Biotechnology and bioengineering, 113, 1719-1728, 2016.
- M. Olkiewicz, Torres, C. M., Jiménez, L., Font, J., & Bengoa, C, ” Scale-up and economic analysis of biodiesel production from municipal primary sewage sludge,” Bioresource technology, 214, 122-131, 2016. https://doi.org/10.1016/j.biortech.2016.04.098
- X. Fan, & Burton, R, “Recent development of biodiesel feedstocks and the applications of glycerol: a review,” Open Fuels & Energy Science Journal, 2, 100-109, 2009. doi: 10.2174/1876973X01002010100
- A. Dermawan, K. Obidzinski, and H. Komarudin, “Withering before full bloom? Bioenergy in Southeast Asia,” CIFOR Working Paper, 2012.
- EU Parliament Directive 2009/28; EC 2009, “EC of the European Parliament and of the Council of 23 April 2009 on the promotion of the use of energy from renewable sources and amending and subsequently repealing Directives 2001/77/EC and 2003/30/EC (Text with EEA relevance),” Official Journal League, 140, 16-62, 2009.
- Y. Su, Zhang, P., & Su, Y,, “An overview of biofuels policies and industrialization in the major biofuel producing countries,” Renewable and Sustainable Energy Reviews, 50, 991-1003, 2015. https://doi.org/10.1016/j.rser.2015.04.032
- U. EPA. Renewable Fuel Standard Program Online. Available: https://www.epa.gov/renewable-fuel-standard-program
- I. R. E. Agency. Adanced Liquid Biofuels Online. Available: https://www.irena.org/-/media/Files /IRENA/Agency/ Publication/ 2016/IRENA_Innovation_Outlook_Advanced_Liquid_Biofuels_2016.pdf
- H. C. Ong, Mahlia, T. M. I., Masjuki, H. H., & Honnery, D,, “Life cycle cost and sensitivity analysis of palm biodiesel production,” Fuel, 98, 131-139, 2012. https://doi.org/10.1016/j.fuel.2012.03.031
- G. Sorda, Banse, M., & Kemfert, C, “An overview of biofuel policies across the world,” Energy policy, 38, 6977-6988, 2010. https://doi.org/10.1016/j.enpol.2010.06.066
- D. Mitchell, “A Note on Rising Food Prices: Policy Research Working Paper 4682,” The World Bank, Washington, D.C., United StatesJuly 2008.
- J. Tomei, & Helliwell, R, “Food versus fuel? Going beyond biofuels,” Land use policy, 56, 320-326, 2016. https://doi.org/10.1016/j.landusepol.2015.11.015
- A. M. Renzaho, Kamara, J. K., & Toole, M,, “Biofuel production and its impact on food security in low and middle income countries: Implications for the post-2015 sustainable development goals,” Renewable and Sustainable Energy Reviews, 78, 503-516, 2017. https://doi.org/10.1016/j.rser.2017.04.072
- J. Beckman, Gooch, E., Gopinath, M., & Landes, M, “Market impacts of China and India meeting biofuel targets using traditional feedstocks,” Biomass and bioenergy, 108, 258-564, 2018. https://doi.org/10.1016/j.biombioe.2017.11.018
- I. C. Macedo, Seabra, J. E., & Silva, J. E, “Green house gases emissions in the production and use of ethanol from sugarcane in Brazil: the 2005/2006 averages and a prediction for 2020,” Biomass and bioenergy, 32, 582-595, 2008. https://doi.org/10.1016/j.biombioe.2007.12.006
- D. Imhoff and C. Badaracoo, “Ethanol: The Farm Bill, Springer, 2019. https://doi.org/10.5822/978-1-61091-975-3_18
- S. Mittlefehldt, “From appropriate technology to the clean energy economy: renewable energy and environmental politics since the 1970s,” Journal of Environmental Studies and Sciences, 8, 212-219, 2018. https://doi.org/10.1007/s13412-018-0471-z
- D. Bentivoglio, & Rasetti, M, “Biofuel sustainability: review of implications for land use and food price,” Italian Review of Agricultural Economics, 70, 7-31, 2015. doi: 10.13128/REA-16975
- E. Riegelhaupt, & Chalico, T. A, Opportunities and challenges for biofuel production in Latin America: a forester’s perspective. Bogor, Indonesia, Center for International Forestry Research (CIFOR), 2009.
- G. Hochman, Scott, Kaplan, Deepak, Rajagopal, David, Zilberman, “Biofuel and food-commodity prices,” Agriculture 2, 272-281, 2012. https://doi.org/10.3390/agriculture2030272
- S. A. Wich, Gaveau, D., Abram, N., Ancrenaz, M., Baccini, A., Brend, S., … & Goossens, B, “Understanding the impacts of land-use policies on a threatened species: is there a future for the Bornean orang-utan?,” PLoS One, 7, e49142, 2012. https://doi.org/10.1371/journal.pone.0049142
- R. Bailey, “Another inconvenient truth: How biofuel policies are deepening poverty and accelerating climate change,” Oxfam Policy and Practice: Climate Change and Resilience, vol. 4, 1-58, 2008.
- J. Couwenberg, Dommain, R., & Joosten, H, “Greenhouse gas fluxes from tropical peatlands in south-east Asia,” Global Change Biology, 16, 1715-1732, 2010.
- A. Hooijer, Page, S., Canadell, J. G., Silvius, M., Kwadijk, J., Wosten, H., & Jauhiainen, J. (2010). . , “Current and future CO2 emissions from drained peatlands in Southeast Asia,” Biogeosciences, 7, 1505-1514, 2010. doi:10.5194/bg-7-1505-2010
- M. R. Guariguata, Masera, O. R., Johnson, F. X., von Maltitz, G., Bird, N., Tella, P., & Martínez-Bravo, R, A review of environmental issues in the context of biofuel sustainability frameworks, 69, Center for International Forestry Research (CIFOR), 2011.
- C. Moser, Hildebrandt, T., & Bailis, R, “International sustainability standards and certification: Sustainable development of biofuels in Latin America and the Caribbean, Springer, 2014.
- S. Naik, V. V. Goud, P. K. Rout, K. Jacobson, and A. K. Dalai, “Characterization of Canadian biomass for alternative renewable biofuel,” Renewable energy, 35, 1624-1631, 2010. https://doi.org/10.1016/j.renene.2009.08.033
- S. Shashank, L. Czarnowska, and M. Bogacka, “The influence of compositions of alternative fuels on higher heating values,” Archives of Waste Management and Environmental Protection, vol. 17, 141-148, 2015.
- T. Raj, M. Kapoor, R. Gaur, J. Christopher, B. Lamba, D. K. Tuli, et al., “Physical and chemical characterization of various Indian agriculture residues for biofuels production,” Energy & Fuels, 29, 3111-3118, 2015.
- A. V. Bridgwater and D. Boocock, An overview of fast pyrolysis of biomass for the production of liquid fuels: Developments in Thermochemical Biomass Conversion, 1/2, Springer Science & Business Media, 2013.
- P. Basu, Pyrolysis and Torrefaction: Biomass gasification, pyrolysis and torrefaction: practical design and theory, Academic press, 2018.
- O. A. Pătrăuanu, L. Lazăr, V. I. Popa, and I. Volf, “Influence of particle size and size distribution on kinetic mechanism of spruce bark polyphenols extraction,” Development, 8, 11, 2019.
- H. Bennadji, K. Smith, M. J. Serapiglia, and E. M. Fisher, “Effect of particle size on low-temperature pyrolysis of woody biomass,” Energy & Fuels, 28, 7527-7537, 2014. https://doi.org/10.1021/ef501869e
- R. Chandra, H. Takeuchi, T. Hasegawa, and V. Vijay, “Experimental evaluation of substrate’s particle size of wheat and rice straw biomass on methane production yield,” Agricultural Engineering International: CIGR Journal, 17, 2015.
- M. Tripathi, J. N. Sahu, and P. Ganesan, “Effect of process parameters on production of biochar from biomass waste through pyrolysis: A review,” Renewable and Sustainable Energy Reviews, vol. 55, 467-481, 2016. https://doi.org/10.1016/j.rser.2015.10.122
- E. Üresin, I. I. Gülsaç, M. S. Budak, M. Ünsal, K. Özgür Büyüksakallı, P. Aksoy, et al., “Effects of operational parameters on bio-oil production from biomass,” Waste Management & Research, 37, 516-529, 2019. https://doi.org/10.1177/0734242X18819192
- J. Fermoso, P. Pizarro, J. M. Coronado, and D. P. Serrano, “Advanced biofuels production by upgrading of pyrolysis bio-oil,” Wiley Interdisciplinary Reviews: Energy and Environment, 6, e245, 2017. https://doi.org/10.1002/wene.245
- D. Pradhan, R. Singh, H. Bendu, and R. Mund, “Pyrolysis of Mahua seed (Madhuca indica)–Production of biofuel and its characterization,” Energy conversion and management, 108, 529-538, 2016. https://doi.org/10.1016/j.enconman.2015.11.042
- P. Fu, S. Hu, J. Xiang, L. Sun, S. Su, and S. An, “Study on the gas evolution and char structural change during pyrolysis of cotton stalk,” Journal of Analytical and Applied Pyrolysis, 97, 130-136, 2012. https://doi.org/10.1016/j.jaap.2012.05.012
- K. Zeng, D. P. Minh, D. Gauthier, E. Weiss-Hortala, A. Nzihou, and G. Flamant, “The effect of temperature and heating rate on char properties obtained from solar pyrolysis of beech wood,” Bioresource technology, 182, 114-119, 2015. https://doi.org/10.1016/j.biortech.2015.01.112
- A. P. Muroyama and P. G. Loutzenhiser, “Kinetic analyses of gasification and combustion reactions of carbonaceous feedstocks for a hybrid solar/autothermal gasification process to continuously produce synthesis gas,” Energy & Fuels, 30, 4292-4299, 2016. https://doi.org/10.1021/acs.energyfuels.6b00359
- M. Pande and A. N. Bhaskarwar, “Biomass conversion to energy: Biomass Conversion, Springer, 2012. https://doi.org/10.1007/978-3-642-28418-2_1
- D. Shen, W. Jin, J. Hu, R. Xiao, and K. Luo, “An overview on fast pyrolysis of the main constituents in lignocellulosic biomass to valued-added chemicals: structures, pathways and interactions,” Renewable and Sustainable Energy Reviews, 51, 761-774, 2015. https://doi.org/10.1016/j.rser.2015.06.054
- P. Roy and G. Dias, “Prospects for pyrolysis technologies in the bioenergy sector: a review,” Renewable and Sustainable Energy Reviews, 77, 59-69, 2017. https://doi.org/10.1016/j.rser.2017.03.136
- E. Cetin, R. Gupta, and B. Moghtaderi, “Effect of pyrolysis pressure and heating rate on radiata pine char structure and apparent gasification reactivity,” Fuel, 84, 1328-1334, 2005. https://doi.org/10.1016/j.fuel.2004.07.016
- R. Kaur, P. Gera, and M. K. Jha, “Study on Effects of Different Operating Parameters on the Pyrolysis of Biomass: A Review,” Journal of Biofuels and Bioenergy (December 2015), 1, 135-147, 2015. doi: 10.5958/2454-8618.2015.00015.2
- A. Debdoubi, A. El Amarti, E. Colacio, M. Blesa, and L. Hajjaj, “The effect of heating rate on yields and compositions of oil products from esparto pyrolysis,” International Journal of Energy Research, 30, 1243-1250, 2006. https://doi.org/10.1002/er.1215
- H. C. Butterman and M. J. Castaldi, “Biomass to fuels: impact of reaction medium and heating rate,” Environmental Engineering Science, 27, 539-555, 2010.
- P. Fu, S. Hu, J. Xiang, L. Sun, S. Su, and J. Wang, “Evaluation of the porous structure development of chars from pyrolysis of rice straw: Effects of pyrolysis temperature and heating rate,” Journal of Analytical and Applied Pyrolysis, 98, 177-183, 2012. https://doi.org/10.1016/j.jaap.2012.08.005
- D. Angın, “Effect of pyrolysis temperature and heating rate on biochar obtained from pyrolysis of safflower seed press cake,” Bioresource technology, 128, 593-597, 2013. https://doi.org/10.1016/j.biortech.2012.10.150
- Q. Lu, W.-Z. Li, and X.-F. Zhu, “Overview of fuel properties of biomass fast pyrolysis oils,” Energy Conversion and Management, 50, 1376-1383, 2009. https://doi.org/10.1016/j.enconman.2009.01.001
- G. Chen, J. Andries, Z. Luo, and H. Spliethoff, “Biomass pyrolysis/gasification for product gas production: the overall investigation of parametric effects,” Energy conversion and management, 44, 1875-1884, 2003. https://doi.org/10.1016/S0196-8904(02)00188-7
- H. Yang, R. Yan, H. Chen, D. H. Lee, D. T. Liang, and C. Zheng, “Pyrolysis of palm oil wastes for enhanced production of hydrogen rich gases,” Fuel Processing Technology, 87, 935-942, 2006. https://doi.org/10.1016/j.fuproc.2006.07.001
- S. Fremaux, S.-M. Beheshti, H. Ghassemi, and R. Shahsavan-Markadeh, “An experimental study on hydrogen-rich gas production via steam gasification of biomass in a research-scale fluidized bed,” Energy conversion and management, vol. 91, 427-432, 2015. https://doi.org/10.1016/j.enconman.2014.12.048
- D. Baruah, D. Baruah, and M. Hazarika, “Artificial neural network based modeling of biomass gasification in fixed bed downdraft gasifiers,” Biomass and bioenergy, 98, 264-271, 2017. https://doi.org/10.1016/j.biombioe.2017.01.029
- G. T. Jeong, Yang, H. S., & Park, D. H, “Optimization of transesterification of animal fat ester using response surface methodology,” Bioresource technology, 100, 25-30, 2009. https://doi.org/10.1016/j.biortech.2008.05.011
- H. N. Bhatti, Hanif, M. A., & Qasim, M, “Biodiesel production from waste tallow,” Fuel 87, 2961-2966, 2008. https://doi.org/10.1016/j.fuel.2008.04.016
- J. Xue, Grift, T. E., & Hansen, A. C, “Effect of biodiesel on engine performances and emissions,” Renewable and Sustainable energy reviews, 15, 1098-1116, 2011. https://doi.org/10.1016/j.rser.2010.11.016
- A. Saydut, Kafadar, A. B., Aydin, F., Erdogan, S., Kaya, C., & Hamamci, C, “Effect of homogeneous alkaline catalyst type on biodiesel production from soybean Glycine max (L.) Merrill oil,” 2016. http://nopr.niscair.res.in/handle/123456789/41020
- E. C. Santos, dos Santos, T. C., Guimarães, R. B., Ishida, L., Freitas, R. S., & Ronconi, C. M, “Guanidine-functionalized Fe3O4 magnetic nanoparticles as basic recyclable catalysts for biodiesel production,” RSC Advances, 5, 48031-48038, 2015. doi: 10.1039/C5RA07331F
- G. Baskar, Gurugulladevi, A., Nishanthini, T., Aiswarya, R., & Tamilarasan, K, “Optimization and kinetics of biodiesel production from Mahua oil using manganese doped zinc oxide nanocatalyst,” Renewable energy, 103, 641-646, 2017. https://doi.org/10.1016/j.renene.2016.10.077
- G. Baskar, & Soumiya, S, “Production of biodiesel from castor oil using iron (II) doped zinc oxide nanocatalyst,” Renewable Energy, 98, 101-107, 2016. https://doi.org/10.1016/j.renene.2016.02.068
- M. R. Avhad, & Marchetti, J. M, Uses of Enzymes for Biodiesel Production: Advanced Bioprocessing for Alternative Fuels, Biobased Chemicals, and Bioproducts, Woodhead Publishing, 2019. https://doi.org/10.1016/B978-0-12-817941-3.00007-3
- J. Thangaraja and C. Kannan, “Effect of exhaust gas recirculation on advanced diesel combustion and alternate fuels-A review,” Applied Energy, 180, 169-184, 2016. https://doi.org/10.1016/j.apenergy.2016.07.096
- J. G. Speight, Thermal Cracking Processes: Heavy Oil Recovery and Upgrading, Gulf Professional Publishing, 2019.
- V. Wiggers, H. Meier, A. Wisniewski Jr, A. C. Barros, and M. W. Maciel, “Biofuels from continuous fast pyrolysis of soybean oil: a pilot plant study,” Bioresource technology, 100, 6570-6577, 2009. https://doi.org/10.1016/j.biortech.2009.07.059
- M. Omidghane, E. Jenab, M. Chae, and D. C. Bressler, “Production of renewable hydrocarbons by thermal cracking of oleic acid in the presence of water,” Energy & Fuels, 31, 9446-9454, 2017. https://doi.org/10.1021/acs.energyfuels.7b00988
- P. Tamunaidu and S. Bhatia, “Catalytic cracking of palm oil for the production of biofuels: optimization studies,” Bioresource Technology, 98, 3593-3601, 2007. https://doi.org/10.1016/j.biortech.2006.11.028
- J. C. Serrano-Ruiz and J. A. Dumesic, “Catalytic routes for the conversion of biomass into liquid hydrocarbon transportation fuels,” Energy & Environmental Science, 4, 83-99, 2011. doi: 10.1039/C0EE00436G
- B. Thangaraj, P. R. Solomon, B. Muniyandi, S. Ranganathan, and L. Lin, “Catalysis in biodiesel production—a review,” Clean Energy, 3, 2-23, 2019. https://doi.org/10.1093/ce/zky020
- A. Demirbas, “Biodiesel production from vegetable oils via catalytic and non-catalytic supercritical methanol transesterification methods,” Progress in energy and combustion science, 31, 466-487, 2005. https://doi.org/10.1016/j.pecs.2005.09.001
- F. R. Amin, H. Khalid, H. Zhang, S. u Rahman, R. Zhang, G. Liu, et al., “Pretreatment methods of lignocellulosic biomass for anaerobic digestion,” Amb Express, 7, 72, 2017. https://doi.org/10.1186/s13568-017-0375-4
- N. S. Talha and S. Sulaiman, “Overview of catalysts in biodiesel production,” ARPN Journal of Engineering and Applied Sciences, 11, 439-48, 2016.
- Z. Guo, B. Liu, Q. Zhang, W. Deng, Y. Wang, and Y. Yang, “Recent advances in heterogeneous selective oxidation catalysis for sustainable chemistry,” Chemical Society Reviews, 43, 3480-3524, 2014. doi: 10.1039/C3CS60282F
- N. Hindryawati, G. P. Maniam, M. R. Karim, and K. F. Chong, “Transesterification of used cooking oil over alkali metal (Li, Na, K) supported rice husk silica as potential solid base catalyst,” Engineering Science and Technology, an International Journal, 17, 95-103, 2014. https://doi.org/10.1016/j.jestch.2014.04.002
- B. Thangaraj, Jia, Z., Dai, L., Liu, D., & Du, W, “Lipase NS81006 immobilized on Fe3O4 magnetic nanoparticles for biodiesel production,” Ovidius University Annals of Chemistry, 27, 13-21, 2016. https://doi.org/10.1515/auoc-2016-0008
- C. Li, H. Wang, Y. Luo, G. Wen, and Z. Jiang, “A novel gold nanosol SERS quantitative analysis method for trace Na+ based on carbon dot catalysis,” Food chemistry, 289, 531-536, 2019. https://doi.org/10.1016/j.foodchem.2019.03.032
- M. Math and K. Chandrashekhara, “Optimization of alkali catalyzed transesterification of safflower oil for production of biodiesel,” Journal of Engineering, 2016, 2016. https://doi.org/10.1155/2016/8928673
- V. T. da Silva and L. A. Sousa, Catalytic upgrading of fats and vegetable oils for the production of fuels: The role of catalysis for the sustainable production of bio-fuels and bio-chemicals, Elsevier, 2013. https://doi.org/10.1016/B978-0-444-56330-9.00003-6
- I. Atadashi, M. K. Aroua, A. A. Aziz, and N. Sulaiman, “The effects of water on biodiesel production and refining technologies: A review,” Renewable and sustainable energy reviews, 16, 3456-3470, 2012. https://doi.org/10.1016/j.rser.2012.03.004
- I. Atadashi, M. K. Aroua, A. A. Aziz, and N. Sulaiman, “Production of biodiesel using high free fatty acid feedstocks,” Renewable and sustainable energy reviews, 16, 3275-3285, 2012. https://doi.org/10.1016/j.rser.2012.02.063
- H. Luo, W. Fan, Y. Li, and G. Nan, “Biodiesel production using alkaline ionic liquid and adopted as lubricity additive for low-sulfur diesel fuel,” Bioresource technology, 140, 337-341, 2013. https://doi.org/10.1016/j.biortech.2012.11.112
- J. Shabaker, G. Huber, and J. Dumesic, “Aqueous-phase reforming of oxygenated hydrocarbons over Sn-modified Ni catalysts,” Journal of Catalysis, 222, 180-191, 2004. https://doi.org/10.1016/j.jcat.2003.10.022
- R. Davda, J. Shabaker, G. Huber, R. Cortright, and J. A. Dumesic, “A review of catalytic issues and process conditions for renewable hydrogen and alkanes by aqueous-phase reforming of oxygenated hydrocarbons over supported metal catalysts,” Applied Catalysis B: Environmental, 56, 71-186, 2005. https://doi.org/10.1016/j.apcatb.2004.04.027
- T. Hirai, N.-o. Ikenaga, T. Miyake, and T. Suzuki, “Production of hydrogen by steam reforming of glycerin on ruthenium catalyst,” Energy & Fuels, 19, 1761-1762, 2005. https://doi.org/10.1021/ef050121q
- F. Yang, M. A. Hanna, and R. Sun, “Value-added uses for crude glycerol–a byproduct of biodiesel production,” Biotechnology for biofuels, 5, 13, 2012. https://doi.org/10.1186/1754-6834-5-13
- B. J. Kerr, W. A. Dozier III, and K. Bregendahl, “Nutritional value of crude glycerin for nonruminants,” in Proceedings of the 68th Minnesota nutrition conference: Modern Concepts In Livestock Production, 220-234, 2007.
- Z. Chi, D. Pyle, Z. Wen, C. Frear, and S. Chen, “A laboratory study of producing docosahexaenoic acid from biodiesel-waste glycerol by microalgal fermentation,” Process Biochemistry, 42, 1537-1545, 2007. https://doi.org/10.1016/j.procbio.2007.08.008
- R. D. Ashby, D. K. Solaiman, and T. A. Foglia, “Bacterial poly (hydroxyalkanoate) polymer production from the biodiesel co-product stream,” Journal of Polymers and the Environment, 12, 105-112, 2004. https://doi.org/10.1023/B:JOOE.0000038541.54263.d9
- G. Mothes, C. Schnorpfeil, and J. U. Ackermann, “Production of PHB from crude glycerol,” Engineering in Life Sciences, 7, 475-479, 2007.
- Y. Liang, Y. Cui, J. Trushenski, and J. W. Blackburn, “Converting crude glycerol derived from yellow grease to lipids through yeast fermentation,” Bioresource technology, 101, 7581-7586, 2010. https://doi.org/10.1016/j.biortech.2010.04.061
- Y. Liang, N. Sarkany, Y. Cui, and J. W. Blackburn, “Batch stage study of lipid production from crude glycerol derived from yellow grease or animal fats through microalgal fermentation,” Bioresource technology, 101, 6745-6750, 2010. https://doi.org/10.1016/j.biortech.2010.03.087
- S. Papanikolaou and G. Aggelis, “Biotechnological valorization of biodiesel derived glycerol waste through production of single cell oil and citric acid by Yarrowia lipolytica,” Lipid technology, 21, 83-87, 2009.
- S. Papanikolaou, S. Fakas, M. Fick, I. Chevalot, M. Galiotou-Panayotou, M. Komaitis, et al., “Biotechnological valorisation of raw glycerol discharged after bio-diesel (fatty acid methyl esters) manufacturing process: production of 1, 3-propanediol, citric acid and single cell oil,” Biomass and bioenergy, 32, 60-71, 2008. https://doi.org/10.1016/j.biombioe.2007.06.007
- G. Baskar, G. Kalavathy, R. Aiswarya, and I. A. Selvakumari, Advances in bio-oil extraction from nonedible oil seeds and algal biomass: Advances in Eco-Fuels for a Sustainable Environment, Elsevier, 2019. https://doi.org/10.1016/B978-0-08-102728-8.00007-3
- M. Quader and S. Ahmed, Bioenergy with carbon capture and storage (BECCS): future prospects of carbon-negative technologies, Elsevier, 2017. https://doi.org/10.1016/B978-0-12-805423-9.00004-1
- A. K. Azad, M. Rasul, M. M. Khan, and S. Sharma, “Macadamia biodiesel as a sustainable and alternative transport fuel in Australia,” Energy Procedia, 110, 543-548, 2017. https://doi.org/10.1016/j.egypro.2017.03.182
- A. Azad, “Biodiesel from mandarin seed oil: a surprising source of alternative fuel,” Energies, 10, 1689, 2017. https://doi.org/10.3390/en10111689
- S. Reham, H. H. Masjuki, M. Kalam, I. Shancita, I. R. Fattah, and A. Ruhul, “Study on stability, fuel properties, engine combustion, performance and emission characteristics of biofuel emulsion,” Renewable and Sustainable Energy Reviews, 52, 1566-1579, 2015. https://doi.org/10.1016/j.rser.2015.08.013
- S. Vellaiyan, “Enhancement in combustion, performance, and emission characteristics of a biodiesel-fueled diesel engine by using water emulsion and nanoadditive,” Renewable Energy, 145, 2108-2120, 2020. https://doi.org/10.1016/j.renene.2019.07.140
- F. Y. Hagos, O. M. Ali, R. Mamat, and A. A. Abdullah, “Effect of emulsification and blending on the oxygenation and substitution of diesel fuel for compression ignition engine,” Renewable and Sustainable Energy Reviews, 75, 1281-1294, 2017. https://doi.org/10.1016/j.rser.2016.11.113
- K. Araújo, D. Mahajan, R. Kerr, and M. d. Silva, “Global biofuels at the crossroads: an overview of technical, policy, and investment complexities in the sustainability of biofuel development,” Agriculture, 7, 32, 2017. https://doi.org/10.3390/agriculture7040032
- J. Lane, “Biofuels mandates around the world,” Biofuels Digest, 3, 2016.
- Z. Wang, S. Wu, Y. Huang, S. Huang, S. Shi, X. Cheng, et al., “Experimental investigation on spray, evaporation and combustion characteristics of ethanol-diesel, water-emulsified diesel and neat diesel fuels,” Fuel, 231, 438-448, 2018. https://doi.org/10.1016/j.fuel.2018.05.129
- T. Yatsufusa, T. Kumura, Y. Nakagawa, and Y. Kidoguchi, “Advantage of using water-emulsified fuel on combustion and emission characteristics,” Fuel, 5, 2009.
- M. Huo, S. Lin, H. Liu, and F. L. Chia-fon, “Study on the spray and combustion characteristics of water–emulsified diesel,” Fuel, 123, 218-229, 2014. https://doi.org/10.1016/j.fuel.2013.12.035
- Z. Wang, S. Shi, S. Huang, J. Tang, T. Du, X. Cheng, et al., “Effects of water content on evaporation and combustion characteristics of water emulsified diesel spray,” Applied energy, 226, 397-407, 2018. https://doi.org/10.1016/j.apenergy.2018.06.023
- C. F. Uzoh, A. Nnuekwe, O. Onukwuli, S. Ofochebe, and E. Chinyere, “Optimal Route for Effective Conversion of Rubber Seed Oil to Biodiesel with Desired Key fuel properties,” Journal of Cleaner Production, 280(1), 124563, 2020. https://doi.org/10.1016/j.jclepro.2020.124563
- Y.-K. Oh, K.-R. Hwang, C. Kim, J. R. Kim, and J.-S. Lee, “Recent developments and key barriers to advanced biofuels: a short review,” Bioresource technology, 257, 320-333, 2018. https://doi.org/10.1016/j.biortech.2018.02.089
- S. Maroa and F. Inambao, Biodiesels Production Proccesses and Technologies: Biodiesel, Combustion, Performance and Emissions Characteristics, Springer, 2020. https://doi.org/10.1007/978-3-030-51166-1_3
- C. J. Chuck, Bannister, C. D., Hawley, J. G., Davidson, M. G., La Bruna, I., & Paine, A, “Predictive model to assess the molecular structure of biodiesel fuel,” Energy & Fuels, 23, 290-2294, 2009. https://doi.org/10.1021/ef801085s
- U. S. Congress, “Energy Independence and Security Act of 2007,” U.S. Congress, Washinton, DC, USA19/12/2007 2007.
- S. K. Hoekman, A. Broch, C. Robbins, E. Ceniceros, and M. Natarajan, “Review of biodiesel composition, properties, and specifications,” Renewable and sustainable energy reviews, 16, 43-169, 2012. https://doi.org/10.1016/j.rser.2011.07.143
- J. K. Kurian, G. R. Nair, A. Hussain, and G. V. Raghavan, “Feedstocks, logistics and pre-treatment processes for sustainable lignocellulosic biorefineries: a comprehensive review,” Renewable and Sustainable Energy Reviews, 25, 205-219, 2013. https://doi.org/10.1016/j.rser.2013.04.019
- Z. Yue, D. Ma, S. Peng, X. Zhao, T. Chen, and J. Wang, “Integrated utilization of algal biomass and corn stover for biofuel production,” Fuel, 168, 1-6, 2016. https://doi.org/10.1016/j.fuel.2015.11.079
- N. R. Baral and A. Shah, “Comparative techno-economic analysis of steam explosion, dilute sulfuric acid, ammonia fiber explosion and biological pretreatments of corn stover,” Bioresource technology, 232, 331-343, 2017. https://doi.org/10.1016/j.biortech.2017.02.068
- T. P. Durrett, C. Benning, and J. Ohlrogge, “Plant triacylglycerols as feedstocks for the production of biofuels,” The Plant Journal, 54, 593-607, 2008. https://doi.org/10.1111/j.1365-313X.2008.03442.x
- A. S. Carlsson, “Plant oils as feedstock alternatives to petroleum–A short survey of potential oil crop platforms,” Biochimie, 91, 665-670, 2009. https://doi.org/10.1016/j.biochi.2009.03.021
- T. Eryilmaz, M. K. Yesilyurt, C. Cesur, and O. Gokdogan, “Biodiesel production potential from oil seeds in Turkey,” Renewable and Sustainable Energy Reviews, 58, 842-851, 2016. https://doi.org/10.1016/j.rser.2015.12.172
- H. Venkatesan and S. Sivamani, “Cotton seed biodiesel as alternative fuel: production and its characterization analysis using spectroscopic studies,” International Journal of Renewable Energy Research (IJRER), 7, 1333-1339, 2017. www.ijrer.org(6085)
- A. Karmakar, S. Karmakar, and S. Mukherjee, “Properties of various plants and animals feedstocks for biodiesel production,” Bioresource technology, 101, 7201-7210, 2010. https://doi.org/10.1016/j.biortech.2010.04.079
- A. B. Fadhil and W. S. Abdulahad, “Transesterification of mustard (Brassica nigra) seed oil with ethanol: purification of the crude ethyl ester with activated carbon produced from de-oiled cake,” Energy conversion and management, 77, 495-503, 2014. https://doi.org/10.1016/j.enconman.2013.10.008
- C. Ciubota-Rosie, J. R. Ruiz, M. J. Ramos, and Á. Pérez, “Biodiesel from Camelina sativa: a comprehensive characterisation,” Fuel, 105, 572-577, 2013. https://doi.org/10.1016/j.fuel.2012.09.062
- E. Mupondwa, X. Li, K. Falk, R. Gugel, and L. Tabil, “Technoeconomic analysis of small-scale farmer-owned Camelina oil extraction as feedstock for biodiesel production: a case study in the Canadian prairies,” Industrial Crops and Products, 90, 76-86, 2016. https://doi.org/10.1016/j.indcrop.2016.05.042
- C. Ilkılıç, S. Aydın, R. Behcet, and H. Aydin, “Biodiesel from safflower oil and its application in a diesel engine,” Fuel processing technology, 92, 356-362, 2011. https://doi.org/10.1016/j.fuproc.2010.09.028
- C. Hamamci, A. Saydut, Y. Tonbul, C. Kaya, and A. Kafadar, “Biodiesel production via transesterification from safflower (Carthamus tinctorius L.) seed oil,” Energy Sources, Part A: Recovery, Utilization, and Environmental Effects, 33, 512-520, 2011.
- J. S. Requena, A. C. Guimaraes, S. Q. Alpera, E. R. Gangas, S. Hernandez-Navarro, L. N. Gracia, et al., “Life Cycle Assessment (LCA) of the biofuel production process from sunflower oil, rapeseed oil and soybean oil,” Fuel Processing Technology, 92, 190-199, 2011. https://doi.org/10.1016/j.fuproc.2010.03.004
- E. M. Visser, D. Oliveira Filho, M. A. Martins, and B. L. Steward, “Bioethanol production potential from Brazilian biodiesel co-products,” Biomass and bioenergy, 35, 489-494, 2011. https://doi.org/10.1016/j.biombioe.2010.09.009
- F. Barontini, M. Simone, F. Triana, A. Mancini, G. Ragaglini, and C. Nicolella, “Pilot-scale biofuel production from sunflower crops in central Italy,” Renewable energy, 83, 954-962, 2015. https://doi.org/10.1016/j.renene.2015.05.043
- T. Issariyakul and A. K. Dalai, “Biodiesel production from greenseed canola oil,” Energy & Fuels, 24, 4652-4658, 2010. https://doi.org/10.1021/ef901202b
- C. Baroi, S. Mahto, C. Niu, and A. K. Dalai, “Biofuel production from green seed canola oil using zeolites,” Applied Catalysis A: General, 469, 18-32, 2014. https://doi.org/10.1016/j.apcata.2013.09.034
- S. Nithya, S. Manigandan, P. Gunasekar, J. Devipriya, and W. Saravanan, “The effect of engine emission on canola biodiesel blends with TiO2,” International Journal of Ambient Energy, 40, 838-841, 2019. https://doi.org/10.1080/01430750.2017.1421583
- P. Verma and M. Sharma, “Review of process parameters for biodiesel production from different feedstocks,” Renewable and Sustainable Energy Reviews, 62, 1063-1071, 2016. https://doi.org/10.1016/j.rser.2016.04.054
- J. L. Solis, A. L. Berkemar, L. Alejo, and Y. Kiros, “Biodiesel from rapeseed oil (Brassica napus) by supported Li 2 O and MgO,” International Journal of Energy and Environmental Engineering, 8, 9-23, 2017. https://doi.org/10.1007/s40095-016-0226-0
- C. M. Fernández, L. Fiori, M. J. Ramos, Á. Pérez, and J. F. Rodríguez, “Supercritical extraction and fractionation of Jatropha curcas L. oil for biodiesel production,” The Journal of Supercritical Fluids, 97, 100-106, 2015. https://doi.org/10.1016/j.supflu.2014.11.010
- D. A. Torres-Rodríguez, I. C. Romero-Ibarra, I. A. Ibarra, and H. Pfeiffer, “Biodiesel production from soybean and Jatropha oils using cesium impregnated sodium zirconate as a heterogeneous base catalyst,” Renewable Energy, vol. 93, 323-331, 2016. https://doi.org/10.1016/j.renene.2016.02.061
- J. Rodrigues, A. Canet, I. Rivera, N. Osório, G. Sandoval, F. Valero, et al., “Biodiesel production from crude Jatropha oil catalyzed by non-commercial immobilized heterologous Rhizopus oryzae and Carica papaya lipases,” Bioresource technology, 213, 88-95, 2016. https://doi.org/10.1016/j.biortech.2016.03.011
- K. Singh, B. Singh, S. K. Verma, and D. Patra, “Jatropha curcas: a ten year story from hope to despair,” Renewable and Sustainable Energy Reviews, 35, 356-360, 2014. https://doi.org/10.1016/j.rser.2014.04.033
- N. A. Musa, G. M. Teran, and S. A. Yaman, “Characterization of coconut oil and its biodiesel,” Journal of Scientific Research and Reports, 9(6), 1-6, 2016. https://doi.org/10.9734/JSRR/2016/22293
- B. Venkatesagowda, E. Ponugupaty, A. M. Barbosa-Dekker, and R. F. Dekker, “The purification and characterization of lipases from Lasiodiplodia theobromae, and their immobilization and use for biodiesel production from coconut oil,” Applied biochemistry and biotechnology, 185, 619-640, 2018. https://doi.org/10.1007/s12010-017-2670-6
- W. Roschat, T. Siritanon, B. Yoosuk, and V. Promarak, “Biodiesel production from palm oil using hydrated lime-derived CaO as a low-cost basic heterogeneous catalyst,” Energy conversion and management, 108, 459-467, 2016. https://doi.org/10.1016/j.enconman.2015.11.036
- A. Johari, B. B. Nyakuma, S. H. M. Nor, R. Mat, H. Hashim, A. Ahmad, et al., “The challenges and prospects of palm oil based biodiesel in Malaysia,” Energy, 81, 255-261, 2015. https://doi.org/10.1016/j.energy.2014.12.037
- V. F. de Almeida, P. J. García-Moreno, A. Guadix, and E. M. Guadix, “Biodiesel production from mixtures of waste fish oil, palm oil and waste frying oil: Optimization of fuel properties,” Fuel Processing Technology, 133, 152-160, 2015. https://doi.org/10.1016/j.fuproc.2015.01.041
- M. Mubarak, A. Shaija, and T. Suchithra, “A review on the extraction of lipid from microalgae for biodiesel production,” Algal Research, 7, 117-123, 2015. https://doi.org/10.1016/j.algal.2014.10.008
- J.-Y. Park, M. S. Park, Y.-C. Lee, and J.-W. Yang, “Advances in direct transesterification of algal oils from wet biomass,” Bioresource Technology, 184, 267-275, 2015. https://doi.org/10.1016/j.biortech.2014.10.089
- N. Yodsuwan, P. Kamonpatana, Y. Chisti, and S. Sirisansaneeyakul, “Ohmic heating pretreatment of algal slurry for production of biodiesel,” Journal of biotechnology, 267, 71-78, 2018. https://doi.org/10.1016/j.jbiotec.2017.12.022
- B. Sajjadi, A. A. A. Raman, and H. Arandiyan, “A comprehensive review on properties of edible and non-edible vegetable oil-based biodiesel: composition, specifications and prediction models,” Renewable and Sustainable Energy Reviews, 63, 62-92, 2016. https://doi.org/10.1016/j.rser.2016.05.035
- D. Singh, D. Sharma, S. Soni, S. Sharma, and D. Kumari, “Chemical compositions, properties, and standards for different generation biodiesels: A review,” Fuel, 253, 60-71, 2019. https://doi.org/10.1016/j.fuel.2019.04.174
- G. Knothe, “Biodiesel and renewable diesel: a comparison,” Progress in energy and combustion science, 36, 364-373, 2010. https://doi.org/10.1016/j.pecs.2009.11.004
- T. Manchanda, R. Tyagi, and D. K. Sharma, “Comparison of fuel characteristics of green (renewable) diesel with biodiesel obtainable from algal oil and vegetable oil,” Energy Sources, Part A: Recovery, Utilization, and Environmental Effects, 40, 54-59, 2018. https://doi.org/10.1080/15567036.2017.1405109
- T. Kalnes, T. Marker, and D. R. Shonnard, “Green diesel: a second generation biofuel,” International Journal of Chemical Reactor Engineering, 5 (1), 2007. https://doi.org/10.2202/1542-6580.1554
- M. Vojtisek-Lom, V. Beránek, P. Mikuška, K. Křůmal, P. Coufalík, J. Sikorová, et al., “Blends of butanol and hydrotreated vegetable oils as drop-in replacement for diesel engines: Effects on combustion and emissions,” Fuel, 197, 407-421, 2017. https://doi.org/10.1016/j.fuel.2017.02.039
- A. Dimitriadis, I. Natsios, A. Dimaratos, D. Katsaounis, Z. Samaras, S. Bezergianni, et al., “Evaluation of a hydrotreated vegetable oil (HVO) and effects on emissions of a passenger car diesel engine,” Frontiers in Mechanical Engineering, 4, 7, 2018. https://doi.org/10.3389/fmech.2018.00007
- M. Mofijur, Rasul, M. G., Hassan, N. M. S., Masjuki, H. H., Kalam, M. A., & Mahmudul, H. M, Assessment of Physical, Chemical, and Tribological Properties of Different Biodiesel Fuels: Clean Energy for Sustainable Development, Academic Press, 2017. https://doi.org/10.1016/B978-0-12-805423-9.00014-4
- U. Rajak, Nashine, P., Singh, T. S., & Verma, T. N, “Numerical investigation of performance, combustion and emission characteristics of various biofuels,” Energy Conversion and Management, 156, 235-252, 2018. https://doi.org/10.1016/j.enconman.2017.11.017
- O. M. Ali, Mamat, R., Masjuki, H. H., & Abdullah, A. A, “Analysis of blended fuel properties and cycle-to-cycle variation in a diesel engine with a diethyl ether additive,” Energy conversion and management, 108, 511-519, 2016. https://doi.org/10.1016/j.enconman.2015.11.035
- C. Choi, G. Bower, and R. D. Reitz, “Effects of biodiesel blended fuels and multiple injections on DI diesel engines,” SAE transactions, 388-407, 1997. https://www.jstor.org/stable/44730687
- A. Monyem, Van Gerpen, J. H., & Canakci, M,, “The effect of timing and oxidation on emissions from biodiesel-fueled engines,” Transactions of the ASAE, 44, 5, 2001. (doi: 10.13031/2013.2301
- J. P. Szybist, & Boehman, A. L, “Behavior of a diesel injection system with biodiesel fuel (No. 2003-01-1039),” SAE Technical Paper, 2003. doi: https://doi.org/10.4271/2003-01-1039
- S. H. Park, S. H. Yoon, and C. S. Lee, “Effects of multiple-injection strategies on overall spray behavior, combustion, and emissions reduction characteristics of biodiesel fuel,” Applied Energy, 88, 88-98, 2011. https://doi.org/10.1016/j.apenergy.2010.07.024
- R. K. Pandey, Rehman, A., & Sarviya, R. M, “Impact of alternative fuel properties on fuel spray behavior and atomization,” Renewable and Sustainable Energy Reviews, 16, 1762-1778, 2012. https://doi.org/10.1016/j.rser.2011.11.010
- S. Maroa and F. Inambao, “The effect of cetane number and oxygen content in the performance and emissions characteristics of a diesel engine using biodiesel blends,” Journal of Energy in Southern Africa, 30(2), 1-13, 2019. doi: http://dx.doi.org/10.17159/2413-3051/2019/v30i2a5337
- S. Lahane, & Subramanian, K. A, “Effect of different percentages of biodiesel–diesel blends on injection, spray, combustion, performance, and emission characteristics of a diesel engine,” Fuel, 139, 537-545, 2015. https://doi.org/10.1016/j.fuel.2014.09.036
- H. J. Kim, Park, S. H., & Lee, C. S, “Impact of fuel spray angles and injection timing on the combustion and emission characteristics of a high-speed diesel engine,” Energy, 107, 572-579, 2016. https://doi.org/10.1016/j.energy.2016.04.035
- A. Atmanli, Ileri, E., Yuksel, B., & Yilmaz, N, “Extensive analyses of diesel–vegetable oil–n-butanol ternary blends in a diesel engine,” Applied energy, 145, 155-162, 2015. https://doi.org/10.1016/j.apenergy.2015.01.071
- G. Knothe, “Improving biodiesel fuel properties by modifying fatty ester composition,” Energy & Environmental Science, 2, 759-766, 2009. doi: 10.1039/B903941D
- O. N. de Freitas, Rial, R. C., Cavalheiro, L. F., dos Santos Barbosa, J. M., Nazário, C. E. D., & Viana, L. H, “Evaluation of the oxidative stability and cold filter plugging point of soybean methyl biodiesel/bovine tallow methyl biodiesel blends,” Industrial Crops and Products, 140, 111667, 2019. https://doi.org/10.1016/j.indcrop.2019.111667
- M. I. Jahirul, Brown, R. J., & Senadeera, W, Correlation Between Physicochemical Properties and Quality of Biodiesel: Application of Thermo-fluid Processes in Energy Systems, Springer Nature, 2018. https://doi.org/10.1007/978-981-10-0697-5_3
- A. Demirbas, Biodiesel: Springer, 2008.
- M. T. Chaichan, & Ahmed, S. T, “Evaluation of performance and emissions characteristics for compression ignition engine operated with disposal yellow grease,” International Journal of Engineering and Science, 2, 111-122, 2013. www.theijes.com
- M. Canakci, & Sanli, H, “Biodiesel production from various feedstocks and their effects on the fuel properties,” Journal of industrial microbiology & biotechnology, 35, 431-441, 2008. https://doi.org/10.1007/s10295-008-0337-6
- E. Buyukkaya, “Effects of biodiesel on a DI diesel engine performance, emission and combustion characteristics,” Fuel, 89, 3099-3105, 2010. https://doi.org/10.1016/j.fuel.2010.05.034
- S. Som, Ramirez, A. I., Longman, D. E., & Aggarwal, S. K, “Effect of nozzle orifice geometry on spray, combustion, and emission characteristics under diesel engine conditions,” Fuel, 90, 1267-1276, 2011. https://doi.org/10.1016/j.fuel.2010.10.048
- J. Heywood, B, Internal combustion engine fundamentals, McGraw-Hill Education (India), 2012.
- A. Atmanli, “Effects of a cetane improver on fuel properties and engine characteristics of a diesel engine fueled with the blends of diesel, hazelnut oil and higher carbon alcohol,” Fuel, 172, 209-217, 2016. https://doi.org/10.1016/j.fuel.2016.01.013
- Y. Devarajan, Munuswamy, D. B., Nagappan, B., & Pandian, A. K, “Performance, combustion and emission analysis of mustard oil biodiesel and octanol blends in diesel engine,” Heat and Mass Transfer, 54, 1803-1811, 2018. https://doi.org/10.1007/s00231-018-2274-x
- J. P. Szybist, Song, J., Alam, M., & Boehman, A. L, “Biodiesel combustion, emissions and emission control,” Fuel processing technology, 88, 679-691, 2007. https://doi.org/10.1016/j.fuproc.2006.12.008
- M. Lapuerta, Rodríguez-Fernández, J., & Armas, O, “Correlation for the estimation of the density of fatty acid esters fuels and its implications. A proposed biodiesel cetane index,” Chemistry and physics of lipids, 163, 720-727, 2010. https://doi.org/10.1016/j.chemphyslip.2010.06.004
- E. G. Giakoumis, & Sarakatsanis, C. K, “Estimation of biodiesel cetane number, density, kinematic viscosity and heating values from its fatty acid weight composition,” Fuel, 222, 574-585, 2018. https://doi.org/10.1016/j.fuel.2018.02.187
- G. Knothe, J. Krahl, and J. Van Gerpen, The biodiesel handbook: Elsevier, 2015.
- A. M. Hochhauser, “Review of prior studies of fuel effects on vehicle emissions,” SAE International Journal of Fuels and Lubricants, vol. 2, 541-567, 2009. https://www.jstor.org/stable/26273409
- R. Sathiyamoorthi, & Sankaranarayanan, G,, “The effects of using ethanol as additive on the combustion and emissions of a direct injection diesel engine fuelled with neat lemongrass oil-diesel fuel blend,” Renewable Energy, 101, 747-756, 2017. https://doi.org/10.1016/j.renene.2016.09.044
- M. Karabektas, Ergen, G., Hasimoglu, C., & Murcak, A, “Performance and emission characteristics of a diesel engine fuelled with emulsified biodiesel-diesel fuel blends,” International Journal of Automotive Engineering and Technologies, 5, 176-185, 2016. https://doi.org/10.18245/ijaet.287183
- M. V. Kumar, Babu, A. V., & Kumar, P. R, “The impacts on combustion, performance and emissions of biodiesel by using additives in direct injection diesel engine,” Alexandria Engineering Journal, 57, 509-516, 2018. https://doi.org/10.1016/j.aej.2016.12.016
- P. Krisanangkura, S. Lilitchan, S. Phankosol, K. Aryusuk, and K. Krisnangkura, “Gibbs energy additivity approaches to QSPR in modelling of isentropic compressibility of biodiesel,” Journal of Molecular Liquids, 249, 126-131, 2018. https://doi.org/10.1016/j.molliq.2017.10.150
- G. Douhéret, M. I. Davis, J. C. R. Reis, and M. J. Blandamer, “Isentropic compressibilities—experimental origin and the quest for their rigorous estimation in thermodynamically ideal liquid mixtures,” ChemPhysChem, 2, 148-161, 2001.
- A. F. Lopes, M. del Carmen Talavera-Prieto, A. G. Ferreira, J. B. Santos, M. J. Santos, and A. T. Portugal, “Speed of sound in pure fatty acid methyl esters and biodiesel fuels,” Fuel, 116, 242-254, 2014. https://doi.org/10.1016/j.fuel.2013.07.044
- D. J. Luning Prak, “Density, viscosity, speed of sound, bulk modulus, surface tension, and flash point of binary mixtures of butylcyclohexane with toluene or n-hexadecane,” Journal of Chemical & Engineering Data, 61, 3595-3606, 2016. https://doi.org/10.1021/acs.jced.6b00516
- M. Lapuerta, J. R. Agudelo, M. Prorok, and A. L. Boehman, “Bulk modulus of compressibility of diesel/biodiesel/HVO blends,” Energy & fuels, 26, 1336-1343, 2012. https://doi.org/10.1021/ef201608g
- P. Kiełczyński, S. Ptasznik, M. Szalewski, A. Balcerzak, K. Wieja, and A. Rostocki, “Thermophysical properties of rapeseed oil methyl esters (RME) at high pressures and various temperatures evaluated by ultrasonic methods,” Biomass and bioenergy, 107, 113-121, 2017. https://doi.org/10.1016/j.biombioe.2017.09.015
- T. Zhan, Y. Zhang, J. Chen, X. Liu, and M. He, “Measurement of the speed of sound in supercritical n–hexane at temperatures from (509.17–637.99) K and pressures from (3.5–7.5) MPa,” Fluid Phase Equilibria, 497, 97-103, 2019. https://doi.org/10.1016/j.fluid.2019.06.001
- L. S. Ott, M. L. Huber, and T. J. Bruno, “Density and speed of sound measurements on five fatty acid methyl esters at 83 kPa and temperatures from (278.15 to 338.15) K,” Journal of Chemical & Engineering Data, 53, 2412-2416, 2008. https://doi.org/10.1021/je8003854
- S. V. Freitas, M. L. Paredes, J.-L. Daridon, Á. S. Lima, and J. A. Coutinho, “Measurement and prediction of the speed of sound of biodiesel fuels,” Fuel, 103, 1018-1022, 2013. https://doi.org/10.1016/j.fuel.2012.09.082
- A. Gautam and A. K. Agarwal, “Determination of important biodiesel properties based on fuel temperature correlations for application in a locomotive engine,” Fuel, 142, 289-302, 2015. https://doi.org/10.1016/j.fuel.2014.10.032
- A. L. Boehman, D. Morris, J. Szybist, and E. Esen, “The impact of the bulk modulus of diesel fuels on fuel injection timing,” Energy & fuels, 18, 1877-1882, 2004. https://doi.org/10.1021/ef049880j
- H. G. How, H. H. Masjuki, M. Kalam, and Y. H. Teoh, “Influence of injection timing and split injection strategies on performance, emissions, and combustion characteristics of diesel engine fueled with biodiesel blended fuels,” Fuel, 213, 106-114, 2018. https://doi.org/10.1016/j.fuel.2017.10.102
- M. E. Tat, & Van Gerpen, J. H, “Measurement of Biodiesel Speed of Sound and Its Impact on Injection Timing,” National Renewable Energy Lab, Golden, Colarado, USA, 2003.
- S. Jaichandar and K. Annamalai, “Experimental Investigation on the Influences of Varying Injection Timing on the Performance of a B20 JOME Biodiesel Fueled Diesel Engine,” Journal of Mechanical Engineering, 14(1), 57-74, 2017.
- D. Qi, M. Leick, Y. Liu, and F. L. Chia-fon, “Effect of EGR and injection timing on combustion and emission characteristics of split injection strategy DI-diesel engine fueled with biodiesel,” Fuel, 90, 1884-1891, 2011. https://doi.org/10.1016/j.fuel.2011.01.016
- C. Sayin, M. Gumus, and M. Canakci, “Effect of fuel injection timing on the emissions of a direct-injection (DI) diesel engine fueled with canola oil methyl ester-diesel fuel blends,” Energy & Fuels, 24, 2675-2682, 2010. https://doi.org/10.1021/ef901451n
- M. E. Tat and J. H. Van Gerpen, “Measurement of Biodiesel Speed of Sound and Its Impact on Injection Timing: Final Report; Report 4 in a Series of 6,” National Renewable Energy Lab., Golden, 2003. https://doi.org/10.2172/15003584
- S. M. Palash, Kalam, M. A., Masjuki, H. H., Arbab, M. I., Masum, B. M., & Sanjid, A, “Impacts of NOx reducing antioxidant additive on performance and emissions of a multi-cylinder diesel engine fueled with Jatropha biodiesel blends,” Energy Conversion and Management, 77, 577-585, 2014. https://doi.org/10.1016/j.enconman.2013.10.016
- R. L. McCormick, Alvarez, J. R., & Graboski, M. S, “NOx solutions for biodiesel,” National Renewable Energy Laboratory, 2003. http://www.osti.gov/bridge
- A. Maghari and M. S. Sadeghi, “Prediction of sound velocity and heat capacities of n-alkanes from the modified SAFT-BACK (of state,” Fluid phase equilibria, 252, 152-161, 2007. https://doi.org/10.1016/j.fluid.2006.12.007
- A. Queimada, J. Coutinho, I. Marrucho, and J.-L. Daridon, “Corresponding-states modeling of the speed of sound of long-chain hydrocarbons,” International journal of thermophysics, 27, 1095-1109, 2006. doi: 10.1007/s10765-006-0105-7
- C. Avendaño, T. Lafitte, C. S. Adjiman, A. Galindo, E. A. Müller, and G. Jackson, “SAFT-γ force field for the simulation of molecular fluids: 2. Coarse-grained models of greenhouse gases, refrigerants, and long alkanes,” The Journal of Physical Chemistry B, 117, 2717-2733, 2013. https://doi.org/10.1021/jp306442b
- M. Alavianmehr, M. El-Shaikh, F. Akbari, and R. Behjatmanesh-Ardakani, “A new (of state for modeling thermodynamic properties of some fatty acids alkyl esters, methyl ester-based biodiesels and their blends,” Fluid Phase Equilibria, 442, 53-61, 2017. https://doi.org/10.1016/j.fluid.2017.03.004
- X. Meng, M. Jia, and T. Wang, “Predicting biodiesel densities over a wide temperature range up to 523 K,” Fuel, 111, 216-222, 2013. https://doi.org/10.1016/j.fuel.2013.04.050
- A. L. Boehman, D. Morris, J. Szybist, and E. Esen, “The impact of the bulk modulus of diesel fuels on fuel injection timing,” Energy & fuels, 18, 1877-1882, 2004. https://doi.org/10.1021/ef049880j
- R. Stephen, Turns, An introduction to combustion: concepts and applications, McGraw Hill Education (India) Private Limited, 2012.
- A. Demirbas, “Prediction of higher heating values for biodiesels from their physical properties,” Energy Sources, Part A: Recovery, Utilization, and Environmental Effects, 31, 633-638, 2009. https://doi.org/10.1080/15567030701750515
- S. Sadrameli, W. Seames, and M. Mann, “Prediction of higher heating values for saturated fatty acids from their physical properties,” Fuel, 87, 1776-1780, 2008. https://doi.org/10.1016/j.fuel.2007.10.020
- A. Demirbas, “Calculation of higher heating values of fatty acids,” Energy Sources, Part A: Recovery, Utilization, and Environmental Effects, 38, 2693-2697, 2016. https://doi.org/10.1080/15567036.2015.1115924
- A. Demirbas, Ak, N., Aslan, A., & Sen, N, “Calculation of higher heating values of hydrocarbon compounds and fatty acids,” Petroleum Science and Technology, 36, 712-717, 2018. https://doi.org/10.1080/10916466.2018.1443126
- A. S. Silitonga, Masjuki, H. H., Mahlia, T. M. I., Ong, H. C., Chong, W. T., & Boosroh, M. H, “Overview properties of biodiesel diesel blends from edible and non-edible feedstock.,” Renewable and Sustainable Energy Reviews, vol. 22, 346-360, 2013. https://doi.org/10.1016/j.rser.2013.01.055
- S. Maroa and F. Inambao, “Cetane Improvers and Ethanol Performance and Emissions Characteristics Using Pyrorated Biodiesel,” in 2018 International Conference on the Industrial and Commercial Use of Energy (ICUE), 1-8, 2018
- M. T. Chaichan, & Ahmed, S. T,, “Effect of fuel cetane number on multi-cylinders direct injection diesel engine performance and exhaust emissions,” Al-Khwarizmi Engineering Journal, 8, 65-75, 2012. http://alkej.uobaghdad.edu.iq/index.php/alkej/article/view/106
- S. Maroa and F. Inambao, Physicochemical Properties of Biodiesel: Biodiesel, Combustion, Performance and Emissions Characteristics, Springer, 2020. https://doi.org/10.1007/978-3-030-51166-1_5
- R. K. Saluja, V. Kumar, and R. Sham, “Stability of biodiesel–A review,” Renewable and Sustainable Energy Reviews, vol. 62, pp. 866-881, 2016. https://doi.org/10.1016/j.rser.2016.05.001
- H. Tang, A. Wang, S. O. Salley, and K. S. Ng, “The effect of natural and synthetic antioxidants on the oxidative stability of biodiesel,” Journal of the American Oil Chemists’ Society, 85, 373-382, 2008. doi 10.1007/s11746-008-1208-z
- X. Chen, Zhang, Y., Zu, Y., Yang, L., Lu, Q., & Wang, W, “Antioxidant effects of rosemary extracts on sunflower oil compared with synthetic antioxidants.,” International Journal of Food Science & Technology, 49, 385-391, 2014.
- E. N. Frankel, “In search of better methods to evaluate natural antioxidants and oxidative stability in food lipids,” Trends in Food Science & Technology, 4, 220-225, 1993. https://doi.org/10.1016/0924-2244(93)90155-4
- R. O. Dunn, “Effect of temperature on the oil stability index (OSI) of biodiesel,” Energy & Fuels, 22, 57-662, 2007. https://doi.org/10.1021/ef700412c
- S. Jain, & Sharma, M. P, “Effect of metal contaminants and antioxidants on the storage stability of Jatropha curcas biodiesel,” Fuel, vol. 109, pp. 379-383, 2013. https://doi.org/10.1016/j.fuel.2013.03.050
- M. A. Fazal, Haseeb, A. S. M. A., & Masjuki, H. H, “Effect of different corrosion inhibitors on the corrosion of cast iron in palm biodiesel,” Fuel Processing Technology, 92, 2154-2159, 2011. https://doi.org/10.1016/j.fuproc.2011.06.012
- E. Saltas, Bouilly, J., Geivanidis, S., Samaras, Z., Mohammadi, A., & Iida, Y, “Investigation of the effects of biodiesel aging on the degradation of common rail fuel injection systems,” Fuel, 200, 357-370, 2017. https://doi.org/10.1016/j.fuel.2017.03.064
- N. Kumar, “Oxidative stability of biodiesel: Causes, effects and prevention,” Fuel, 190, 328-350, 2017. https://doi.org/10.1016/j.fuel.2016.11.001
- S. R. Westbrook, Fuels for land and marine diesel engines and for non-aviation gas turbines: Significance of tests for petroleum products, ASTM International, 2003.
- M. Angelovič, Jablonický, J., Tkáč, Z., & Angelovič, M, “Oxidative stability of fatty acid alkyl esters: a review,” Potravinarstvo Slovak Journal of Food Sciences, 9, 417-426, 2015. DOI: https://doi.org/10.5219/500
- M. A. Fazal, Jakeria, M. R., Haseeb, A. S. M. A., & Rubaiee, S, “Effect of antioxidants on the stability and corrosiveness of palm biodiesel upon exposure of different metals,” Energy, 135, 220-226, 2017. https://doi.org/10.1016/j.energy.2017.06.128
- L. M. Das, Bora, D. K., Pradhan, S., Naik, M. K., & Naik, S. N, “Long-term storage stability of biodiesel produced from Karanja oil,” Fuel, 88, 2315-2318, 2009. https://doi.org/10.1016/j.fuel.2009.05.005
- T. K. Jose, & Anand, K, “Effects of biodiesel composition on its long term storage stability,” Fuel, 177, 190-196, 2016. https://doi.org/10.1016/j.fuel.2016.03.007
- G. El Diwani, & El Rafie, S, “Modification of thermal and oxidative properties of biodiesel produced from vegetable oils,” International Journal of Environmental Science & Technology, vol. 5(3), 391-400, 2008.
- M. A. Kalam, Masjuki, H. H., Cho, H. M., Mosarof, M. H., Mahmud, M. I., Chowdhury, M. A., & Zulkifli, N. W. M, “Influences of thermal stability, and lubrication performance of biodegradable oil as an engine oil for improving the efficiency of heavy duty diesel engine,” Fuel, 196, 36-46, 2017. https://doi.org/10.1016/j.fuel.2017.01.071
- G. El Diwani, El Rafie, S., & Hawash, S, “Protection of biodiesel and oil from degradation by natural antioxidants of Egyptian Jatropha,” International Journal of Environmental Science & Technology, 6, 369-378, 2009.
- M. Nadeem, Abdullah, M., Javid, A., Arif, A. M., & Mahmood, T, “Evaluation of functional fat from interesterified blends of butter oil and Moringa oleifera oil,” Pakistan Journal of Nutrition, 11, 725, 2012.
- M. Serrano, Martínez, M., & Aracil, J, “Long term storage stability of biodiesel: influence of feedstock, commercial additives and purification step,” Fuel processing technology, 116, 135-141, 2013. https://doi.org/10.1016/j.fuproc.2013.05.011
- J. Bacha, Freel, J., Gibbs, A., Gibbs, L., Hemighaus, G., Hoekman, K., … & Lesnini, D, “Diesel fuels technical review,” Chevron Global Marketing, 1-116, 2007.
- K. Wadumesthrige, Ara, M., Salley, S. O., & Ng, K. S, “Investigation of lubricity characteristics of biodiesel in petroleum and synthetic fuel,” Energy & Fuels, 23, 2229-2234, 2009. https://doi.org/10.1021/ef800887y
- J. Hu, Du, Z., Li, C., & Min, E, “Study on the lubrication properties of biodiesel as fuel lubricity enhancers,” Fuel, 84, 1601-1606., 2005. https://doi.org/10.1016/j.fuel.2005.02.009
- F. Sundus, Fazal, M. A., & Masjuki, H. H, “Tribology with biodiesel: A study on enhancing biodiesel stability and its fuel properties,” Renewable and Sustainable Energy Reviews, 70, 399-412, 2017. https://doi.org/10.1016/j.rser.2016.11.217
- M. Lapuerta, Sánchez-Valdepeñas, J., Bolonio, D., & Sukjit, E “Effect of fatty acid composition of methyl and ethyl esters on the lubricity at different humidities,” Fuel, 184, 202-210, 2016. https://doi.org/10.1016/j.fuel.2016.07.019
- R. Lanjekar and D. Deshmukh, “A review of the effect of the composition of biodiesel on NOx emission, oxidative stability and cold flow properties,” Renewable and Sustainable Energy Reviews, 54, 1401-1411, 2016. https://doi.org/10.1016/j.rser.2015.10.034
- H. Imahara, Minami, E., & Saka, S, “Thermodynamic study on cloud point of biodiesel with its fatty acid composition,” Fuel, 85, 1666-1670, 2006. https://doi.org/10.1016/j.fuel.2006.03.003
- M. I. Arbab, Masjuki, H. H., Varman, M., Kalam, M. A., Imtenan, S., & Sajjad, H, “Fuel properties, engine performance and emission characteristic of common biodiesels as a renewable and sustainable source of fuel,” Renewable and Sustainable Energy Reviews, 22, 133-147, 2013. https://doi.org/10.1016/j.rser.2013.01.046
- L. Gouveia, Oliveira, A. C., Congestri, R., Bruno, L., Soares, A. T., Menezes, R. S., & Tzovenis, I, Biodiesel from microalgae: Biodiesel from microalgae. In Microalgae-based biofuels and bioproducts, Woodhead Publishing, 2017. https://doi.org/10.1016/B978-0-08-101023-5.00010-8
- J. M. Dias, Alvim-Ferraz, M. C., & Almeida, M. F, “Comparison of the performance of different homogeneous alkali catalysts during transesterification of waste and virgin oils and evaluation of biodiesel quality,” Fuel, 87, 3572-3578, 2008. https://doi.org/10.1016/j.fuel.2008.06.014
- R. G.Hedden, Bioheat: Bioenergy, A. Dahiya, Ed., Academic Press, 2015.
- M. Bockisch, Analytical Methods, Fats and Oils Handbook, Elsevier, 2015.
- S. Adhikari, Nam, H., & Chakraborty, J. P, Conversion of Solid Wastes to Fuels and Chemicals Through Pyrolysis: Waste Biorefinery, Elsevier 2018. https://doi.org/10.1016/B978-0-444-63992-9.00008-2
- N. K. Patel, & Shah, S. N, Biodiesel from Plant Oils: Food, energy, and water, Elsevier, 2015. https://doi.org/10.1016/B978-0-12-800211-7.00011-9
Citations by Dimensions
Citations by PlumX
Google Scholar
Scopus
Crossref Citations
- Kennedy C Onyelowe, Ahmed M Ebid, Michael E Onyia, Ezenwa C Amanamba, "Estimating the swelling potential of non-carbon–based binder (NCBB)-treated clayey soil for sustainable green subgrade using AI (GP, ANN and EPR) techniques." International Journal of Low-Carbon Technologies, vol. 17, no. , pp. 807, 2022.
- Deepali T. Marghade, Vivek P. Bhange, Jagdish W. Gabhane, "Aquatic Weeds as Bioenergy Feedstock." In Novel Feedstocks for Biofuels Production, Publisher, Location, 2022.
No. of Downloads Per Month
No. of Downloads Per Country

