Impact of Salinity on Stabilized Leachate Treatment from Ozonation Process
Volume 5, Issue 6, Page No 1511-1516, 2020
Author’s Name: Iva Yenis Septiariva1, I Wayan Koko Suryawan2, Novi Kartika Sari3,a), Ariyanti Sarwono1
View Affiliations
1Sanitary Engineering Laboratory, Study Program of Civil Engineering, Faculty of Engineering, Universitas Sebelas Maret, Jalan Ir Sutami 36A, Kentingan, Surakarta, Indonesia
2Department of Environmental Engineering, Faculty of Infrastructure Planning, Universitas Pertamina, Komplek Universitas Pertamina, Jalan Sinabung II, Terusan Simprug, Jakarta 12220, Indonesia
3Environmental Engineering Study Program, Jurusan Teknologi Infrastruktur dan Kewilayahan, Institut Teknologi Sumatera, Jl. Terusan Ryacudu, Way Huwi, Kec. Jati Agung, Kabupaten Lampung Selatan, Lampung 35365, Indonesia
a)Author to whom correspondence should be addressed. E-mail: novi.sari@tl.itera.ac.id
Adv. Sci. Technol. Eng. Syst. J. 5(6), 1511-1516 (2020); ![]() DOI: 10.25046/aj0506181
DOI: 10.25046/aj0506181
Keywords: Salinity, Ozone pretreatment, Leachate, Biodegradability
Export Citations
Leachate generated from landfill Indonesia is typical has high salinity and organic matters during the dry season, potentially contaminating water and the environment. An increase in salinity might deteriorate the activity of the microorganisms on the decomposition process. This research aims to study the feasibility of the ozonation technique for treating stabilized leachate. The parameters tested were pH, COD, BOD5 BOD5/COD ratio, and TDS. Independent variables of this study were salinity with different concentration from ±0.4 ppt; ±5 ppt; ±10 ppt; and ±20 ppt and ozone contact time up to 60 minutes with an ozone dose of 600 mg/hour. Experimental results revealed that salinity ranging from 0.4 ppt to 20 ppt had little effect on COD removal, and the removal efficiency was around 30%. Further, the ozonation process exhibited better organic removal with an improved BOD5 concentration from 426 mg/L to 887 mg/L in low salinity conditions (0.4 ppt). Meanwhile, it significantly affected the old leachate treatment, where the leachate biodegradability can be enhanced from 0.120 (initial condition) to 0.321 with salt addition of 20 ppt.
Received: 28 September 2020, Accepted: 29 November 2020, Published Online: 21 December 2020
I am text block. Click edit button to change this text. Lorem ipsum dolor sit amet, consectetur adipiscing elit. Ut elit tellus, luctus nec ullamcorper mattis, pulvinar dapibus leo.
1. Introduction
The end of life of municipal solid waste (MSW) in landfills has always been expected because of its cost and operational efficiency [1]. Landfill leachate results from percolated rainwater and moisture passing through the layers of wastes in landfills [2]. The combination of pollutant parameters like BOD5 (Biochemical Oxygen Demand), COD (Chemical Oxygen Demand), ammonia, and inorganic salts makes leachate a potential contamination source in river water and surface water. The characteristics and composition of leachate depend on the biological and chemical processes that occur during waste degradation, solid waste composition, rainfall density, groundwater percolation rate, and landfill age.
Government regulation requires minimizing the risk to public health, therefore all of the leachates generated from conventional landfills must be treated according to quality standards. The BOD5/COD ratio can predict changes in the biodegradation rate in leachate. In general, the ratio of BOD5/COD contained in leachate ranges from 0.4 – 0.6. This ratio range states that the organic compounds contained in leachate are biodegradable [3]. One of the causes of the low degradability of leachate is the high salinity value, where salinity is one of the inhibitors in the degradation process of microorganisms.
Lim et al. stated that salinity does not affect the hydrolysis and acid formation processes, but inhibits bacteria’s activity carrying out the digestion process [4]. The effect of salinity on the waste degradation process can be measured through the COD content, the characteristics of the biogas produced, and methane gas production. Leachate salinity contain potassium (K+), sodium (Na+) and chloride (Cl–) ions [5]. Chloride inhibits bacterial activity so that it can reduce COD content. High salinity content can reduce methane gas formation [6]. Due to the high complexity in its waste composition and characteristics, it is arduous to define a better leachate treatment strategy [7].
Apart from having a high salt concentration, leachate concentrate also contains large amounts of refractory organics [8]. In addition, old leachate consists primarily of refractory organics due to less volatile fatty acids, which is turn can lower the BOD5/COD ratio [9]. The physical-chemical process can be applied to pretreatment, tertiary processing, and the overall leachate treatment process. The purpose of the oxidation process in leachate processing is to oxidize organic substances to the most stable form of oxidation, namely into carbon dioxide and water, and increase the biodegradability of organic pollutants suitable for economic, biological processing [10] Furthermore, in principle, physicochemical technologies are suitable for wastewater treatment with extremely low biodegradability, such as stabilized leachate [11] Ozone treatment is one of the strongest chemical processes in leachate processing, because it has the potential for oxidation to reduce stubborn organic fractions.
However, the variation of salinity in landfill leachate fluctuates, leading to further deteriorating treatment efficiency [12]. Several studies have reported on the influence of high salinity on the landfill leachate by the ozonation process [13-15]. This research was designed to examine the treatment performance subjected to different salinity by the ozonation process from stabilized landfill leachate by adopting those approaches. The scope of future research is to compare three types of leachate characteristics, namely young leachate, semi-old leachate, and stabilized leachate with variations in the concentration of salinity by processing with ozonation.
2. Material and Method
2.1. Properties of leachate
Mature leachate was obtained from an aged landfill in Batam City, Indonesia, which has been operating since 1992. This research was conducted in a research laboratory at Universal University in Batam City. Research sampling was carried out at the inlet section of the leachate treatment plant, namely the landfill in Batam City. The laboratory test results from sampling on the landfill are leachate belonging to the characteristics of old leachate which has a COD concentration of 3000-4000 mg/L.
The mature or stabilized leachate’s COD concentration was 3,000-4,000 mg/L, ammonia concentration was 1,000-1,500 mg/L, and pH was 7.8. The leachate was categorized as stabilized leachate for this study due to the exceedingly low BOD5/COD ratio (<0.15) and high salinity content. The leachate was pumped from the leachate collecting pond. After collection, the stabilized leachate was stored in a cool container (4°C) to maintain the inherent characteristics. Before subjected to the ozonation process, the leachate samples were prefiltered using a vacuum pump.
2.2. Laboratory scale reactor
An illustration of leachate treatment with ozonation can be seen in Figure 1. It is equipped with the ozone generator that can supply ozone with a flow rate of 0.25 g/hour in the frequency of 50/60 Hz, providing an ozone flow rate reaching 1.4 L/minute after calibration [9] . KI solution was prepared to absorb excess ozone gas and safety. The ozone concentration was determined according to the standard method by titration using KI solution. The processes in the reactor were run in a thoroughly complete mix condition with 10 L volume of the reactor.
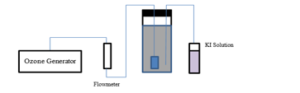
Figure 1: Schematic diagram of ozonation reactor
In Figure 1, a flow diagram of the leachate treatment process in the ozone reactor can be described. The first step is to prepare a KI (Potassium Iodide) solution by dissolving some potassium iodide powder with aquadest. Then, the solution was put into an iron tube with a tube and put into the ozone reactor. Stabilized leachate was put into the ozone reactor at a volume of 10 L. After that, prepared the flowmeter by adjusting the flowrate to 1.4 L / minute. The flowmeter was mounted on the ozone generator and connected to an ozone reactor which already contains old leachate.
2.3. Experimental procedure
The physicochemical treatment process in this study involved ozonation used in the batch operation. At the start of each experiment, the reactor was filled with 9 L of stabilized leachate. Samples were regularly taken at the specified time of 20 minutes, accounting for different applied salinity concentrations. These conditions were performed at a salt concentration of 0.4 ppt, 5 ppt, 10 ppt, and 20 ppt for 60 minutes. The ozonation for treating stabilized landfill leachate was operated for 20 minutes, 40 minutes, and 60 minutes, respectively. Different salt concentrations were adjusted by adding NaCl.
2.4. Sampling and analysis
The initial leachate quality was all measured to determine the original characteristics of the contaminants in the leachate. The initial characteristic parameters consisted of pH, temperature, conductivity, COD, BOD5, and TDS as for leachate. The leachate used was certainly not low in salinity (as a control).
The leachate properties were analyzed for the following elements: COD, BOD5, and TDS. The ozonation reactor was continuously monitored (online measuring of pH), and sampling was taken (COD, BOD5, and BOD5/COD ratio) during every cycle. Samples collected every operational cycle were filtered through a 0.45 µm filter before analysis. All the parameters were analyzed based on the procedure BOD5 (APHA A-B 1995), COD (APHA B-C 1995), TSS (APHA N 1995), TKN (APHA C 1995), and salinity (APHA B&C 1995) [16].
3. Results and Discussion
3.1. Leachate characteristics
The collected leachates’ initial characteristics depend on the municipal solid waste’s waste composition and water content (MSW). The quality of stabilized leachate has not fulfilled the effluent standard, set by government regulation, and both are presented in Table 1. It can be ascertained that the salinity in leachate is only 0.4 ppt to be used as a control in research.
Table 1 shows that the untreated stabilized leachate sample possesses a high concentration of chemical parameters compared to government regulation. The leachate sample’s pH value was beyond the quality standards because anaerobic decomposition was attributed to decreased volatile fatty acid concentration. The electrical conductivity value (19.33 mS/cm) demonstrates dissolved inorganic materials in the samples. The concentration of TDS (4,100 mg/L) also fluctuates widely. The leachate’s dark brown colors are mainly caused by ferrous oxide ferric form and the ferric hydroxide colloid formation and complexes with fulvic and humic substances [17]. The stabilized leachate has COD and BOD5 concentration of 3,561 mg/L and 426 mg/L, respectively. This result reveals that organic matter in stabilized leachate is difficult to degrade biologically due to the complexity of organic compounds. The lower BOD5/COD ratio in this leachate characteristic might be attributed to the leachate’s biologically recalcitrant fraction.
Table 1: A comparison of untreated characteristics of the stabilized leachate and leachate quality standards
| Parameter | Units | Stabilized Leachate | Government Regulation |
| pH | 9.4 | 6.0 – 9.0 | |
| Temperature | oC | 24.5 | |
| Conductivity | mS/cm | 19.3 | |
| COD | mg/L | 3,561.0 | 300 |
| BOD5 | mg/L | 426.0 | 150 |
| BOD5/COD | 0.12 | ||
| TKN | mg/L | 1,757.0 | 60 |
| Salinity | ppt | 0.4 | |
| TDS | mg/L | 4,100.0 |
3.2. Stabilized leachate treatment: Effect of ozone contact time and salinity
The chemical oxidation process for the leachate treatment results in a better quality of stabilized leachate than the initial effluent. Ozonation with different salt concentrations contributes to the degradation of COD substances, the improvement of biodegradability ratio, the change in pH, and total dissolved solids.
3.3. The degradation of COD substances
Figure 2 compares the COD reduction in raw leachate at different salinity subjected to ozone treatment at a certain period. From the COD concentration obtained, the oxidation efficiency decreased slightly after 60 minutes. With 20 minutes of ozone exposure time, COD concentration decreased from 3,561 mg/L to 2,538 mg/L and 2,863 mg/L at salinity of 0.4 ppt and 20 ppt respectively. By adjusting the salt concentration of 5 ppt, the COD efficiency removal increases from 19.91% to 31.99% in ozonation time from 40 min to 60 min. Figure 2 shows that as ozone contact time increases, the COD content of stabilized leachate decreases faster than that of initial mature leachate. The initial COD value (3,561 mg/L) declines to 2,479 mg/L, 2,422 mg/L, 2,521 mg/L, and 2,434 mg/L respectively at the ozone contact time of 60 min and different salinity resulting in removal efficiencies of 30.38%, 31.99%, 29.21% and 31.65%.
These results suggested that COD removal efficiencies remained largely unaffected due to variations in salinity from 0.4 ppt to 20 ppt. Moreover, a minor decline in COD content removal was obtained when the ozone contact time was prolonged from 20 min to 60 min. Salinity variations showed a negligible effect on organics removal, while it influenced the nitrification and denitrification efficiency to a more considerable extent. The salt inhibition can be diminished significantly after the long acclimatization time of the biomass [18].
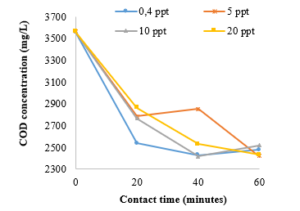
Figure 2: Effect of ozone contact time and the salinity on the COD concentration
The ozonation process and the further oxidation process by accelerating the decomposition of ozone to OH, is one of the wise efforts to be effective in treating leachate. The ozone decomposition reaction is
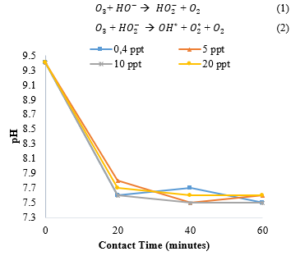
Figure 3: Effect of ozone contact time and the salinity variation on pH
3.4. The change of pH
Applying ozonation at a high leachate pH of 9.4 will most probably lead to a faster ozonation decomposition, therefore a significant enhancement of HO* production which is a more reactive compared to ozone itself, maybe achieved [19]. It is possible that at lower pH, organic pollutants are shifted toward their non-ionized forms. This promotes a lower reactivity toward ozone compared to the ionized or dissociated form [20]. The conversions of the recalcitrant compound will also alter the occurring pH. The pH gradually decreased as ozone contact time increased, and the variation of effluent pH is depicted in Figure 3. When the salt concentration was 0.4 ppt, effluent pH slowly declined from 9.4 to 7.5 as ozonation time was increased from 0 to 60 min. Furthermore, salts of 5 ppt effluent pH gradually decreased from 9.4 to 7.6 after 60 min of ozone contact time. This might be due to the degradation of macromolecular organic content by ozone to form aliphatic acids, alcohols, and aldehydes and subsequently lead to lower pH.
3.5. The improvement of the biodegradability ratio
From Figure 4, it can be seen that ozonation leads to an increase in the BOD5/COD ratio. On the other hand, biodegradability changes one indicator to evaluate the ozone’s efficiency to treat wastewater [21].
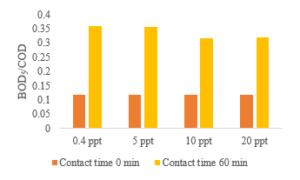
Figure 4: Effect of ozone contact time and the salinity variation on BOD5/COD ratio of stabilized leachate
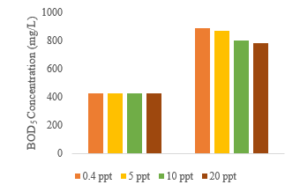
Figure 5: Effect of ozone contact time and the salinity variation on BOD5 concentration of stabilized leachate
The improvement of biodegradability as measured by the BOD5/COD ratio are shown in Figure 4. For a 60 min ozone contact time, COD concentration gradually decreased, and BOD5 concentration slowly increased from 426 mg/L to 865 mg/L at a salinity concentration of 5 ppt (Figure 5). When salinity concentration is adjusted to 20 ppt, biodegradability rises from 0.12 to 0.321. Furthermore, the effect of ozonation is visible at the salinity of 0.4 ppt, and the biodegradability is improved from 0.12 to 0.358. These results indicate that ozonation technology can destruct macromolecular organics into lower molecular weight substances [22, 23] Consequently, the ozonation process yielded a more favorable effluent for biological treatment with a BOD5/COD ratio of more than 0.3 [24].
Advanced treatment with ozone/persulfate oxidation lowered pH from herbal decoction pieces wastewater and improved biodegradability from 0.16 to 0.55 [25]. Another study with ozone/hydrogen peroxide also enhanced the biodegradability of textile wastewater [22, 23] and color removal [26], as well as the efficiency of biological wastewater treatment [27]. Figure 6 shows that there is a close relationship between salinity and biodegradability values. The higher the salinity in leachate, the non-biodegradable food will be processed biologically.
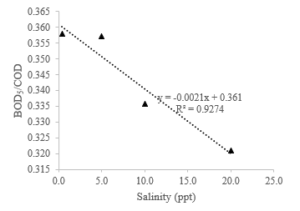
Figure 6: Correlation of ozone salinity and BOD5/COD ratio of stabilized leachate
3.6. Total dissolved solids (TDS) analysis
Leachate generally contains high salinity and TDS. The TDS values of samples subjected to ozone treatment are presented in Figure 7. In Figure 7, the initial concentration of total dissolved solids (TDS) ranged from 4,000 – 7,000 mg/L depending on salt concentrations. TDS in leachate shows the presence of inorganic salts, organic compounds dissolved in leachate. The higher the salinity due to the higher TDS can increase leachate’s toxicity because it can change the ion composition [28]. TDS can be sodium (salt), calcium, magnesium, potassium, carbonate, nitrate, bicarbonate, chloride, and sulfate [29].
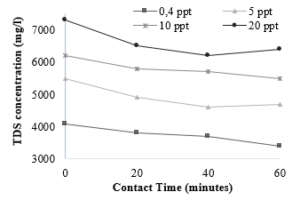
Figure 7: Effect of ozone contact time and the salinity variation on the TDS concentration
TDS primarily revealed the presence of inorganic salts and dissolved organics. The increase in salinity provokes an increase in TDS concentration. The final TDS concentrations increase for all different salinity from 0.4 ppt to 20 ppt. The amount of TDS reflects the availability of the mineralization process and becomes one of the parameters considered for the licensing discharge of landfill leachate. It can be detected in Fig.4 that following the ozone treatment for 60 min of ozone contact time at each salt concentration, the TDS values are 3,400 mg/L, 4,700 mg/L, 5,500 mg/L, and 6,400 mg/L respectively. Overall, ozonation does not significantly contribute to the reduction of TDS.
4. Conclusions
The influence of different salinity conditions on the ozonation of stabilized landfill leachate was studied for several physicochemical characteristics such as COD, BOD5, pH, and TDS. In this project, ozonation technology was employed to treat stabilized leachate to study the COD removal efficiencies at different salinity concentrations. However, the COD value in the stabilized leachate is still above the discharge standard in conformance with the Indonesian regulation. The results indicated that significant removal of the COD parameter could achieve 31.65% for 60 minutes of ozone contact time with 20 ppt salinity. The estimated COD removal in all conditions (0.4 ppt, 5 ppt, and 10 ppt of salinity variation) was 30.38%, 31.99%, and 29.21%, respectively. Though slight difference of COD removal was obtained, biodegradability, as indicated by BOD5/COD ratio, can be significantly enhanced by ozonation.
- Q. Xu, G. Siracusa, S. Di Gregorio and Q. Yuan, “COD removal from biologically stabilized landfill leachate using Advanced Oxidation Processes (AOPs),” Process safety and environmental protection, 120, 278-285, 2018, https://doi.org/10.1016/j.psep.2018.09.014.
- R. Poblete, I. Oller and M. I. C. E. Maldonado, “Improved landfill leachate quality using ozone, UV solar radiation, hydrogen peroxide, persulfate and adsorption processes,” Journal of environmental management, 232, 45-51, 2019, https://doi.org/10.1016/j.jenvman.2018.11.030.
- G. Tchobanoglous, H. Theisen and S. Vigil, Integrated solid waste management: Engineering principles and management, New Jersey: McGraw-Hill, 1993.
- Y. G. Lim, C. Niwa, N. Nagao and T. Toda, “Solubilization and methanogenesis of blue mussels in saline mesophilic anaerobic biodegradation,” International Biodeterioration & Biodegradation, 61(3), 251-260, 2008, https://doi.org/10.1016/j.ibiod.2007.06.012.
- M. Loncnar, M. Zupančič, P. Bukovec and M. Z. Justin, “Fate of saline ions in a planted landfill site with leachate recirculation,” Waste management, 30(1), 110-118, 2010, https://doi.org/10.1016/j.wasman.2009.09.010.
- S. Supparattanapan, P. Saenjan, C. Quantin, J. L. Maeght and O. Grünberger, “Salinity and organic amendment effects on methane emission from a rain-fed saline paddy field,” Soil Science and Plant Nutrition, 55(1), 142-149, 2009, https://doi.org/10.1111/j.1747-0765.2008.00330.x.
- M. Y. Kılıç, K. Kestioglu and T. Yonar, “Landfill leachate treatment by the combination of physicochemical methods with adsorption process,” J. Biol. Environ. Sci, 1(1), 37-43, 2007.
- Wu. Chu, Chen. W, Gu. Zhepei and Li. Qibin, “A Review of the Characteristics of Fenton and Ozonation Systems in Landfill Leachate Treatment,” Science of the Total Environment, Journal Pre-proof, 2020, https://doi.org/10.1016/j.scitotenv.2020.143131.
- I.Y. Septiariva, T. Padmi, E. Damanhuri, and Q. Helmy, “A study on municipal leachate treatment through a combination of biological processes and ozonation,” MATEC Web of Conferences 276, 06030, 2019, https://doi.org/10.1051/matecconf/201927606030.
- X. Li, W. Zhu, Y. Wu, C. Wang, J. Zheng, K. Xu and J. Li, “Recovery of potassium from landfill leachate concentrates using a combination of cation-exchange membrane electrolysis and magnesium potassium phosphate crystallization,” Separation and Purification Technology, 144,1-7,2015,https://doi.org/10.1016/j.seppur.2015.01.035.
- W. Chen, Z. Gu, P. Wen and Q. Li, “Degradation of refractory organic contaminants in membrane concentrates from landfill leachate by a combined coagulation-ozonation process,” Chemosphere, 217, 411-422, 2019, https://doi.org/10.1016/j.chemosphere.2018.11.002.
- O. Lefebvre and R. .. Moletta, “Treatment of organic pollution in industrial saline wastewater: a literature review,” Water research, 40(20), 3671-3682, 2006, https://doi.org/10.1016/j.watres.2006.08.027.
- I. Monje-Ramirez and M. O. De Velasquez, “Removal and transformation of recalcitrant organic matter from stabilized saline landfill leachates by coagulation–ozonation coupling processes,” Water research, 38(9), 2359-2367, 2004, https://doi.org/10.1016/j.watres.2004.02.011.
- W. Chen and Q. Li, “Elimination of UV-quenching substances from MBR-and SAARB-treated mature landfill leachates in an ozonation process: A comparative study,” Chemosphere, 242, 125256, 2020, https://doi.org/10.1016/j.chemosphere.2019.125256.
- S. A. Amr, H. A. Aziz, M. S. Hossain and M. J. Bashir, “Simultaneous removal of COD and color from municipal landfill leachate using Ozone/Zinc sulphate oxidation process,” Glob Nest J,19 (19), 498-504, 2017.
- American Public Health Association, American Water Works Association, Water Pollution Control Federation, & Water Environment Federation, Standard methods for the examination of water and wastewater, 2, USA: American Public Health Association, 1915.
- W. Chen, Z. Gu, P. Wen and Q. Li, “Degradation of refractory organic contaminants in membrane concentrates from landfill leachate by a combined coagulation-ozonation process,” Chemosphere, 217, 411-422, 2019, https://doi.org/10.1016/j.chemosphere.2018.11.002.
- E. R. Rene, S. J. Kim and H. S. Park, “Effect of COD/N ratio and salinity on the performance of sequencing batch reactors,” Bioresource technology, 99(4), 839-846, 2008, https://doi.org/10.1016/j.biortech.2007.01.037.
- M. Chys, V. Oloibiri, W. Audenaert, K. Demeestere and S. W. Van Hulle, “Ozonation of biologically treated landfill leachate: efficiency and insights in organic conversions,” Chemical engineering journal, 277, pp. 104-111, 2015, https://doi.org/10.1016/j.cej.2015.04.099.
- W. Audenaert, D. Vandierendonck, S. H. Van Hulle and I. Nopens, “Comparison of ozone and HO induced conversion of effluent organic matter (EFOM) using ozonation and UV/H2O2 treatment,” Water research, 47(7), 2387-2398, 2013, https://doi.org/10.1016/j.watres.2013.02.003.
- P. Fu, X. Lin, G. Li, Z. Chen and H. Peng, “Degradation of thiol collectors using ozone at a low dosage: kinetics, mineralization, ozone utilization, and changes of biodegradability and water quality parameters,” Minerals, 8, (11), p. 477, 2018, https://doi.org/10.3390/min8110477.
- I. W. K. Suryawan, G. Prajati, A. S. Afifah and M. R. Apritama, “NH3-N and COD reduction in Endek (Balinese textile) wastewater by activated sludge under different DO condition with ozone pretreatment,” Walailak Journal of Science and Technology (WJST), 2020, http://wjst.wu.ac.th/index.php/wjst/article/view/9127.
- I. Suryawan, M. J. Siregar, G. Prajati and A. S. Afifah, “Integrated ozone and anoxic-aerobic activated sludge reactor for endek (Balinese textile) wastewater treatment,” Journal of Ecological Engineering, 20(7), 2019, http://dx.doi.org/10.12911/22998993/109858.
- M. Á. López-Ramírez, O. P. Castellanos-Onorio, M. A. Susunaga-Miranda, F. Lango-Reynoso, M. del Refugio Castañeda-Chávez and J. Montoya-Mendoza, “Treatment of Leachates of a Controlled Landfill in Veracruz By Using the Fenton Method.,” Nature environment & pollution Technology, 18(1), 2019.
- G. Tang, W. Chen, Y. Wei, T. Shao, M. Zhang, Z. Jia and F. Ma, “Novel Advanced Treatment of Physically Treated Effluent from Herbal Decoction Pieces Wastewater Using a Combined Ozone/Persulfate-UBAF Process,” Polish journal of environmental studies, 28(4), 2857-2866, 2019, https://doi.org/10.15244/pjoes/92126.
- N. H. T. Huynh, P. H. Duong and Y. S. .. Yoon, ” A New Combination Treatment System of Ozonation and Electrocoagulation for CI Acid Red 114 Dye Removal from Dyeing Wastewater,” Nature environment and pollution technology, 17(4), 1383-1390, 2018.
- W. Na, “Impact of Ultrasonication-Ozonation Pretreatment on Anaerobic Digestion of Sewage Sludge,” Nature environment and pollution technology, 17(2), 539-542, 2018.
- P. K. Weber-Scannell and L. K. Duffy, “Effects of total dissolved solids on aquatic organism: a review of literature and recommendation for salmonid species,” American Journal of Environmental Sciences, 3(1), 1-6, 2007.
- W. Chen, A. Zhang, G. Jiang and Q. Li, “Transformation and degradation mechanism of landfill leachates in a combined process of SAARB and ozonation,” Waste management, 85, 283-294, 2019, https://doi.org/10.1016/j.wasman.2018.12.038
