Monte Carlo Estimation of Dose in Heterogeneous Phantom Around 6MV Medical Linear Accelerator
Volume 5, Issue 3, Page No 478–486, 2020
Adv. Sci. Technol. Eng. Syst. J. 5(3), 478–486 (2020);
 DOI: 10.25046/aj050359
DOI: 10.25046/aj050359
Keywords: Monte Carlo, MLC, GATE, Artefacts, PTO, Heterogeneous, ECLIPSE, AAA
In this work, we completed a validation of the Varian Clinac IX equipped with the High Definition Multi-Leaf Collimator (HD 120 MLC) instead of the removable jaws, using GATE Monte Carlo Platform version 8.2. We validated the multileaf collimator (MLC) geometry by simulating two dosimetric functions (Percentage Depth Dose (PDD) and Dose Profile (DP)), for 6MV photon beam energy and different field sizes (3×3, 4×4, 6×6, 8×8, 10×10, 12×12, 15×15, and 20×20 cm²). We then compared the results with measurements realized with two detectors, namely the cylindrical ionization chamber and the micro-diode PTW silicon. By applying the Relative Dose Difference method (RDD), we noted a less than 2% and 1% agreement for the field sizes (10×10, 12×12, 15×15, 20×20 cm²) and (3×3, 4×4, 6×6, 8×8 cm²) respectively. Moreover, to evaluate the relevance of Monte Carlo method in a heterogeneous media, particularly in small field sizes (1×1, 2×2, 3×3 cm²), we have simulated three clinical studies based on the Physical Test Objects (PTOs) that are the equivalent slabs of lung and bone included in a water phantom. We noticed that the simulated PDDs exhibit two significant irregularities in the interface between water and lung. To eliminate these phenomena, we have used the “setMaxStepSizeInRegion” parameter implemented in GATE. We also noticed an important difference of 5% corresponding to the small field sizes, between homogeneous and heterogeneous simulated PDDs. We used the RDD method in this case as well. Moreover, we observed a difference between 1-4% between the simulated PDDs and the calculated ones by ECLIPSE Treatment Planning System (TPS). These results indicate that GATE (8.2) is useful in dosimetry with heterogeneous situations as well such as bone and lung.
1. Introduction
In clinical radiotherapy, most TPS are calibrated in a homogeneous media with densities equal to 1. However, some organs have strong heterogeneities such as bone and lung. Hence a better precision requires a corrective dose in conventional TPS. In this context, Monte Carlo simulations present a real alternative allowing enhanced precision related to the transport of high energy photons, particularly in heterogeneous media. However, complex MC simulations require a great amount of computing resources and are time-consuming. Consequently, the optimization of the computation time is necessary. In our study, we used lung and bone equivalent slabs included in a water phantom as Physical Test Objects (PTOs).
Moreover, modern radiotherapy also uses complex beam shapes. For this purpose, we modeled a Varian Clinac IX 6MV photon beam energy with the High Definition Multi-Leaf Collimator (HD 120 MLC). This instrument can hold up to 120 pairs of leaves that move independently to allow the output of a complex beam shape. In practice, there are three types of MLCs, namely type A (e.g. Scanditronix and Siemens) [1], type B (e.g. Elekta) [2] and type C (e.g. Varian) [3]. The three are distinguished by their leaf’s size, speed of movement, and the transmission factor related to their arrangement and their geometry.
In this study, we have used the PTOs to evaluate the relevance of the MC method in the case of small radiation fields used in the context of small tumors. We realized this objective in two parts. In the first part, we have modeled a Varian Clinac IX 6MV photon beam energy to take into account the MLC based on our previous work [4] and using the geometric data provided by the manufacturer [3]. Thus, we compared the simulated dosimetric functions (PDD and DP) for different field sizes (3×3, 4×4, 6×6, 8×8, 10×10, 12×12, 15×15 and 20×20 cm²) to the measured ones using the RDD method [5]. In the second part, we have simulated three PTOs geometries (water+bone, water+lung, and water+bone+lung). Then we compared the PDDs of the heterogeneous media with the homogeneous ones. We also compared the simulated PDDs with the ones calculated by ECLIPSE TPS, based on the Anisotropic Analytic Algorithm (AAA). Finally, we’ve been interested in the optimization of the artefact phenomenon at the interfaces.
2. Material and Methods
2.1. Measurements including the HD 120 MLC
In this study, the Varian Clinac IX 6MV photon beam we used is equipped with the High-Definition Multi-Leaf Collimator (HD 120 MLC). PDD measurements were made at 100 cm of the Source Surface Distance (SSD), with a pitch of 0.1 mm in a depth between 0 and 5 cm in water and 1 mm for depths greater than 5 cm. In the case of DP, we chose 0.1 mm as a pitch in the penumbra region. In measurements with fields greater than or equal to 4x4cm² a cylindrical ionization chamber (Exradin type A28), with a volume of 0.125 cm3 was used, while for fields less than 4x4cm2, measurements were carried out using a micro-diode silicon detector PTW with a volume of 0.03 cm3 placed in a Doseview standard 3D solid water phantom of Standard Imaging.
2.2. HD 120 MLC modelling
The geometry and components materials of the HD 120 MLC have been implemented in GATE (V8.2) code using the manufacturer’s data [3]. It is formed by two blocks (A and B) that can hold 60 independent leaves oriented according to the Y-axis. Each block holds 28 external leaves «half leaves» (0.5 cm width) and 32 internal «quarter leaves» (0.25 cm width). They both are placed at 100 cm from the source [6,7]. Furthermore, the ends of the leaves are rounded with a 16 cm radius, their thickness is 6.9 cm and they are spaced from each other with a distance of 0.0047 cm.
The 32 internal leaves are positioned according to an alternative pattern (Figure 2): a drop with its fine end oriented towards the source (or “Target leaf”) then its neighbor whose fine end is oriented this time towards the isocenter of the accelerator (or «Isocenter leaf»), these two types of leaves differ by the distance tongue and groove which is worth 0.1 and 0.01 cm respectively to create a vertical play between the tongue of a leaf and the groove of the adjacent leaf [8].
Besides, in GATE (V8.2), we introduced four types of leaves (quarter isocenter, quarter target, half isocenter, and half target) (Figure 2). Which we located them at the origin of the marker placed in the entrance of the photon target. Then the leaves were repeated with the possibility of rotation and translation around their center and Y axis, and X, Y, and Z respectively. Figure 3 shows the GATE model of the Varian Clinac IX, including the HD 120 MLC [8].
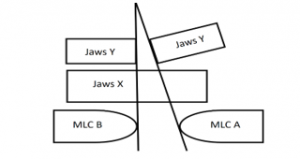
Figure 1: Varian Clinac MLC illustration
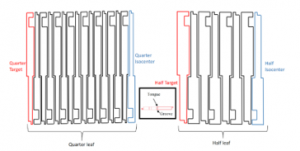
Figure 2: Schematic presentation of the HD 120 MLC, with Target and Isocenter leaf for each type
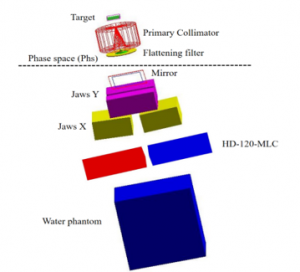
Figure 3: Varian Clinac IX accelerator head GATE modeled including the HD 120 MLC
On the other hand, in GATE (V8.2), the dosimetric functions (PDD and DP) corresponding to the different field sizes (3×3, 4×4, 6×6, 8×8, 10×10, 12×12, 15×15 and 20×20 cm²) were realized using the “DOSE ACTOR”. PDD was normalized at depth (Dmax) where the deposited dose is maximal. We compared the simulation results with those measured using the RDD method [6]. The latter consists of evaluating the relative dose difference between an experimental value and a theoretical reference value using equation 1. The dose difference should be less than 3% in the build-up region and less than 1% for most depths ranging from maximum dose depth (Dmax) to 30 cm. In equation 1, Dc is the calculated absorbed dose, and Dm is the measured dose (reference). Indeed, to obtained good statistic 9.109 particles were generated from a phase space (Phs) previously used as a source [4] and directed into a water phantom of the same size used in measurements.
![]()
Table 1: Material characteristics for lung and bone equivalent slabs
| Materials | Density (g/cm3) | Width (cm) | Composition (%) |
| Lung slab | 0.31 | 10 | H (8.31), C (60.08), N (2.71), O (23.04),
Mg(4.8), Cl(1.02) |
| Bone slab | 1.91 | 5 | H (3.30), C (25.37), N (0.91), O (35.28),
Mg(3.36), P (8.82), Cl(0.03), Ca(22.91) |
2.3. Heterogeneity Study
In this work, we conducted a study of heterogeneity using the two PTOs (lung and bone equivalent slabs). The three heterogeneous geometries studied are illustrated in Figure 4. The Phantom 1 includes a lung equivalent slab with a size of (30 x 30 x 10 cm3) placed at 5 cm from the entrance. The Phantom 2 contains a slab bone with a size of (30 x 30 x 5 cm3) placed at 5 cm. The Phantom 3 includes two slabs: bone and lung located at 5 cm and 10 cm respectively.
To evaluate the ability of GATE to predict the dose distribution in a heterogeneous media, we compared the simulated PDDs with the three phantoms with a homogenous one. This concern six different fields sizes (1×1, 2×2, 3×3, 10×10, 15×15 and 20×20 cm²). In GATE, the composition and the density of lung and bone equivalent slabs were given by the manufacturer (Table 1). We compared GATE results with the ones obtained with the ECLIPSE TPS by applying the RDD method. We note that we used the same GATE geometry in ECLIPSE and that calculations were performed using the AAA algorithm [9,10].
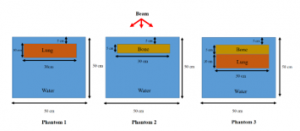
Figure 4: Two PTOs geometries using for three different studies
3. Results and Discussion
3.1. Static validation of the HD 120 MLC
Figures 5 and 6 show the simulated PDDs and DPs compared to those measured for the field sizes (3×3, 4×4, 6×6, 8×8, 10×10, 12×12, 15×15, and 20×20 cm²). Table 2 illustrates the results of this comparison. We note that the PDD results exhibit an agreement of less than 2% for the most points, while with the DP results the differences are around 1% in the build-up region and 2% outside. These simulations indicate that the accuracy with MLC is better than with the removable jaws used in our previous work [4].
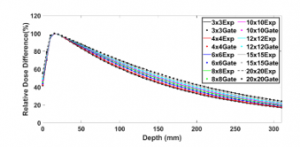
Figure 5: PDDs defined by the 120 HD MLC for different field sizes
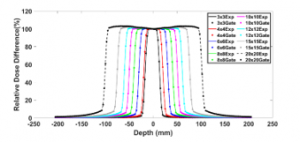
Figure 6: DPs defined by the 120 HD MLC for different field sizes
3.2. Heterogeneity study
- Artefact phenomenon
In the literature, few studies were interested in the artefact phenomenon observed in MC simulations at the boundary between two biological matters [11], for example between water and lung. For this purpose, we simulated the PDD using phantom 1. The results indicated two significant irregularities in the interface between water and lung (Figure 7). The “setMaxStepSizeInRegion” is the key factor for this phenomenon provided in GATE. It is defined as the maximal step size of charged particles. Thus, we performed simulations by varying this parameter within the range of 10-50 μm. Table 3 shows the calculation time for each value corresponding to a step of 10 μm. Adjusting the “setMaxStepSizeInRegion” with the recommended cutoff value [4], the two artefacts are gone.
Table 2: Average RDD between (PDD & DP) calculated and measured ones for different field sizes
| Field sizes (cm2) | RDD (%) | |
| PDD | DP | |
| 3×3 | 0.1666 | 0.1952 |
| 4×4 | 0.1836 | 0.1936 |
| 6×6 | 0.258 | 0.4256 |
| 8×8 | 0.3839 | 0.8934 |
| 10×10 | 0.5289 | 0.9612 |
| 12×12 | 0.7145 | 1.0142 |
| 15×15 | 0.9236 | 1.0958 |
| 20×20 | 1.0147 | 1.3541 |
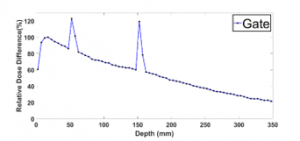
Figure 7: Artefact phenomenon in the boundaries between Water and Lung equivalent slab for 10×10 cm² field size
Table 3: CPU timing for “”setMaxStepSizeInRegion” values
| “setMaxStepSizeInRegion” value (μm) | CPU timing (h) |
| 10 | 30 |
| 20 | 20 |
| 30 | 48 |
| 40 | 53 |
| 50 | 59 |
- Heterogeneity study compared to homogenous one
In Figure 8, the fact that the lung has a weaker density, this doesn’t lead to any change in the PDD in region 1 therefore, the two PDD curves (water and water-lung) are almost identical for all field sizes studied in this region. In region 2, the photon’s attenuation is weaker, and the fact that in small fields (1×1, 2×2, and 3×3 cm²) there is almost no lateral electron equilibrium, lung PDDs are lower than the one in water. This electronic disequilibrium is due to the Compton effect [12]. Indeed, when the electron range produced by the Compton Effect is half of the field size, the electrons produced will transfer their energies outside the radiation field from where the electronic balance is lost. However, for a large field sizes (10×10, 15×15 and 20×20 cm²) the photons’ attenuation and the lateral electron equilibrium becomes significant. This is due to the field sizes increase, consequently, lung PDDs become relatively higher than the ones in the water. Indeed, in region 3, the fact that for all field sizes, the PDDs in the lung are relatively higher than ones in the water, is mainly due to the lower density of lung in region 2.

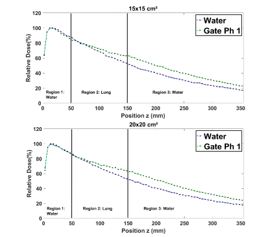
Figure 8: Comparison between the PDD in a homogenous phantom with phantom 1 for 1×1, 2×2, 3×3, 10×10, 15×15 and 20×20 cm² field sizes.
Figure 9 shows that in region 1, for all field sizes the presence of bone seems to not affect the PDDs curves. In region 2, although bone has a higher attenuation coefficient, the related PDDs seem to be relatively weaker than the ones in the water. This is observed for all field sizes, owing to the Compton scattering effect [12]. Indeed, in region 3, the higher attenuation coefficient of bone in region 2, makes that PDDs in bone are still relatively weaker than the ones in water for all field sizes.
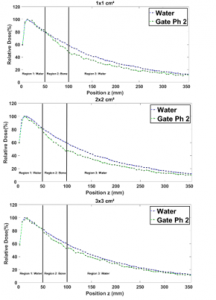
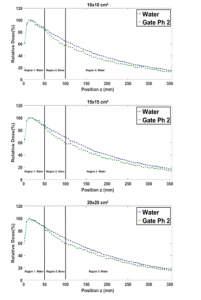
Figure 9: Comparison between the PDD in a homogenous phantom with phantom 2 for 1×1, 2×2, 3×3, 10×10, 15×15 and 20×20 cm² field sizes.
Figure 10 shows that in region 1 the two PDDs curves are almost identical, despite the presence of the two bone and lung slabs. In regions 2 and 3, the related PDDs seem to be relatively weaker than the ones in the water, owing to the Compton scattering effect and the low density of lung. This is observed for all field sizes. In region (4) the fact that for all field sizes, the PDDs in the presence of lung and bone are relatively higher than the ones in water alone, results primarily to the low density of lung in region 3.
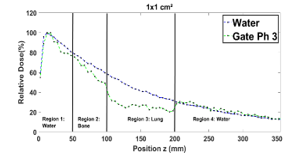

Figure 10: Comparison between the PDD in a homogenous phantom with phantom 3 for 1×1, 2×2, 3×3, 10×10, 15×15 and 20×20 cm² field sizes.
- Heterogeneity comparison study between GATE and ECLIPSE (AAA)
Figure 11 shows that the PDDs obtained by ECLIPSE and GATE from Phantom 1 are in general closely similar with a difference less than 1%, except for the 1×1 cm² field where ECLIPSE PDD exceeds GATE by 4.02%. This can be explained by the fact that ECLIPSE does not take into account the lateral electronic equilibrium in lung slab for very small fields. Table 4 presents the average RDD of the PDDs calculated by ECLIPSE and simulated by GATE for Phantom 1.
Table 4: Average RDD between GATE and ECLIPSE (AAA) PDDs for different field sizes in phantom 1
| Field sizes (cm²) | RDD (%) |
| 1×1 | 4.02 |
| 2×2 | 0.83 |
| 3×3 | 1.27 |
| 10×10 | 1.68 |
| 15×15 | 0.53 |
| 20×20 | 0.63 |
Figure 12 shows that in region 1, the PDDs obtained from Phantom 2, are in general closely similar for all field sizes. In region 2, we note a difference of 1 to 5% explained by the fact that ECLIPSE overestimates the energy deposited by secondary electrons in bone slab [13]. Table 5 presents the average RDD of the PDDs for Phantom 2.
Table 5: Average RDD between GATE and ECLIPSE (AAA) PDDs for different field sizes in phantom 2
| Field sizes (cm²) | RDD (%) |
| 1×1 | 0.26 |
| 2×2 | 1.31 |
| 3×3 | 2.31 |
| 10×10 | 4.08 |
| 15×15 | 4.03 |
| 20×20 | 3.75 |
Figure 13 shows that in region 1, the PDDs obtained from Phantom 2, are in general closely similar for all field sizes. In region 2, for small field sizes, ECLIPSE PDDs exceed the GATE ones by more than 4%. This is because ECLIPSE overestimates the energy deposited by secondary electrons in bone slab [13]. On the other hand, for field sizes greater than or equal to (10×10 cm²) the deference is 2%, owing to the presence also of lung in region 3. Table 6 presents the average difference for PDDs calculated and measured by applying the RDD method.
Table 6: Average RDD between GATE and ECLIPSE (AAA) PDDs for different field sizes in phantom 3
| Field sizes (cm²) | RDD (%) |
| 1×1 | 4.23 |
| 2×2 | 4.59 |
| 3×3 | 3.61 |
| 10×10 | 2.00 |
| 15×15 | 1.91 |
| 20×20 | 1.74 |

Figure 11: Comparison between GATE and ECLIPSE (AAA) PDDs in phantom 1 for 1×1, 2×2, 3×3, 12×12, 15×15 and 20×20 cm² field sizes.

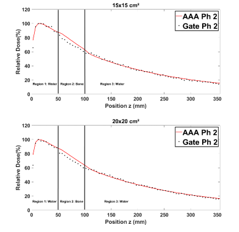
Figure 12: Comparison between GATE and ECLIPSE (AAA) PDDs in phantom 2 for 1×1, 2×2, 3×3, 12×12, 15×15 and 20×20 cm² field sizes.
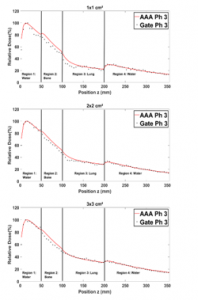
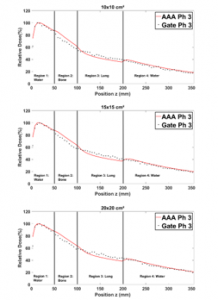
Figure 13: Comparison between GATE and ECLIPSE (AAA) PDDs in phantom 3 for 1×1, 2×2, 3×3, 12×12, 15×15 and 20×20 cm² field sizes.
4. Conclusion
In addition to our previous work, in this study, we successfully used the up-to-date version of GATE 8.2 to simulate the High Definition Multi-Leaf Collimator (HD 120 MLC) using the manufacturers’. We performed the MLC validation by comparing the dosimetric functions (PDD and DP) measured in a water phantom with those simulated for different field sizes using, the relative dose difference method (RDD). Results show an agreement of less than 2% between simulated and measured functions. On the other hand, we demonstrated by using three studies based on the Physical Test Object (PTOs), the capacity of GATE 8.2 code to reproduce the dosimetric function in heterogeneous media such as lung and bone. Moreover, we showed that GATE exceeds ECLIPSE in the assessment of dose in heterogeneous media, since the latter does not take into account the lateral electronic disequilibrium. Indeed, the optimization of the “setMaxStepSizeInRegion” parameter in GATE led to eliminate the phenomenon of artefact in the interface between lung and bone. In conclusion, the static validation of our MLC 120 HD model, as well as the results of the dose distribution in heterogeneous media will lead us in future research for a dynamic validation to test the feasibility of the clinical application of the Monte Carlo simulations.
Conflict of Interest
We declare that we have no conflict of interest.
- AAPM. Basic applications of multileaf collimators. report of the AAPM radiation therapy committee Task Group No 50 AAPM Report No 72 (Madison, WI: American Institute of Physics by Medical Physics Publishing). 2001.
- Heath, E, Seuntjens, J. Development and validation of a BEAMnrc component module for accurate Monte Carlo modelling of the Varian dynamic Millennium multileaf collimator. Phys Med Biol 2003;48(24):4045-4063. https://doi.org/10.1088/0031-9155/48/24/004
- Michael K. Fix, Werner Volken, Daniel Frei, Daniel Frauchiger, Ernst J. Born and Peter Manser, Monte Carlo implementation, validation, and characterization of a 120 leaf MLC. Med. Phys., 38(10) : 5311-5320, 2011. https://doi.org/10.1118/1.3626485
- Z. Aitelcadi, A. Bannan, R. El baydaoui, MR. Mesradi, A. Halimi, S. Elmadani, Feasibility of external radiotherapy dose estimation in homogenous phantom using monte carlo modeling. JATIT, 98(8) :1151-1162, 2020.
- Cho S.H., Vassiliev O.N., Lee S., Liu H.H., Ibbott G.S., and Mohan R. Reference photon dosimetry data and reference phase space data for the 6 mv photon beam from varian clinac 2100 serie linear accelerators. Med. Phys., 32(1) :137–148, 2005. https://doi.org/10.1118/1.1829172
- Huq, MS, Das, IJ, Steinberg, T, Galvin, JM. A dosimetric comparison of various multileaf collimators. Phys Med Biol 2002;47(12):N159-170. https://doi.org/10.1088/0031-9155/47/12/401
- J. V. Siebers, P. J. Keall, J. O. Kim, and R. Mohan, “A method for photon beam Monte Carlo multileaf collimator particle transport,” Phys. Med. Biol. 47(17), 3225 (2002). https://doi.org/10.1088/0031-9155/47/17/312
- C. Borges, M. Zarza-Moreno, E. Heath, N. Teixeira and P. Vaz, Monte Carlo modeling and simulations of the High Definition (HD120) micro MLC and validation against measurements for a 6MV beam. Med.Phys., 39(1), 2012. https://doi.org/10.1118/1.3671935
- Fogliata, A, Vanetti, E, Albers, D, et al. On the dosimetric behaviour of photon dose calculation algorithms in the presence of simple geometric heterogeneities: comparison with Monte Carlo calculations. Phys Med Biol 2007;52(5):1363-1385. https://doi.org/10.1088/0031-9155/52/5/011
- Panettieri, V, Barsoum, P, Westermark, M, Brualla, L, Lax, I. AAA and PBC calculation accuracy in the surface build-up region in tangential beam treatments. Phantom and breast case study with the Monte Carlo code PENELOPE. Radiother Oncol 2009;93(1):94-101. https://doi.org/10.1016/j.radonc.2009.05.010
- Poon, E., Verhaegen, F., 2005. Accuracy of the photon and electron physics in GEANT4 for radiotherapy applications. J. Med. Phys., 2005, 32(6), 1696–1711. https://doi.org/10.1118/1.1895796
- L.A.R. da Rosa, S.C. Cardoso, L.T. Campos, V.G.L. Alves, D.V.S. Batista, A.Facure, Percentage depth dose evaluation in heterogeneous media using thermolumiescent dosimetry. JOURNAL OF APPLIED CLINICAL MEDICAL PHYSICS, 11(1), 2010, 117-127. https://doi.org/10.1120/jacmp.v11i1.2947
- Cardoso SC, Alves VGL, da Rosa LAR, Campos LT, Batista DVS, Facure A, Monte Carlo Simulation of Bony Heterogeneity Effects on Dose Profile for Small Irradiation Field in Radiotherapy. PLoS ONE. 2010; 5(5): e10466. https://doi.org/10.1371/journal.pone.0010466
- Mahdi Madani, El-Bay Bourennane, Safwan El Assad, "Hardware and Secure Implementation of Enhanced ZUC Steam Cipher Based on Chaotic Dynamic S-Box", Advances in Science, Technology and Engineering Systems Journal, vol. 10, no. 1, pp. 37–47, 2025. doi: 10.25046/aj100105
- Pontsho Penelope Mokgatla, Ramadhan Wanjala Salim, Julius Ndambuki, "Enhancing Compressive Strength of Pervious Concrete for Use as Pavement Layer in Urban Roads Aper", Advances in Science, Technology and Engineering Systems Journal, vol. 9, no. 1, pp. 49–66, 2024. doi: 10.25046/aj090106
- Yu-Jin An, Ha-Young Oh, Hyun-Jong Kim, "Forecasting Bitcoin Prices: An LSTM Deep-Learning Approach Using On-Chain Data", Advances in Science, Technology and Engineering Systems Journal, vol. 8, no. 3, pp. 186–192, 2023. doi: 10.25046/aj080321
- El Hadji Malick Ndoye, Ousmane Diallo, Nadir Hakem, Emmanuel Nicolas Cabral, "Interference-Aware Nodes Deployment of a LoRa-Based Architecture for Smart Agriculture in the Southern Region of Senegal", Advances in Science, Technology and Engineering Systems Journal, vol. 7, no. 6, pp. 248–255, 2022. doi: 10.25046/aj070628
- Ming Fong Sie, Jingze Wu, Seth Austin Harding, Chien-Lung Lin, San-Tai Wang, Shih-wei Liao, "Secured Multi-Layer Blockchain Framework for IoT Aggregate Verification", Advances in Science, Technology and Engineering Systems Journal, vol. 7, no. 3, pp. 106–115, 2022. doi: 10.25046/aj070312
- Caglar Arslan, Selen Sipahio?lu, Emre ?afak, Mesut Gözütok, Tacettin Köprülü, "Comparative Analysis and Modern Applications of PoW, PoS, PPoS Blockchain Consensus Mechanisms and New Distributed Ledger Technologies", Advances in Science, Technology and Engineering Systems Journal, vol. 6, no. 5, pp. 279–290, 2021. doi: 10.25046/aj060531
- Hung-Ming Chi, Liang-Yu Chen, Tzu-Chien Hsiao, "Extraction of Psychological Symptoms and Instantaneous Respiratory Frequency as Indicators of Internet Addiction Using Rule-Based Machine Learning", Advances in Science, Technology and Engineering Systems Journal, vol. 6, no. 5, pp. 203–212, 2021. doi: 10.25046/aj060522
- Hsuan-Yu Kuo, Jau-Jr Lin, "Development of Miniaturized Monolithic Isolated Gate Driver", Advances in Science, Technology and Engineering Systems Journal, vol. 6, no. 5, pp. 177–184, 2021. doi: 10.25046/aj060520
- Randy Kuang, Dafu Lou, Alex He, Alexandre Conlon, "Quantum Secure Lightweight Cryptography with Quantum Permutation Pad", Advances in Science, Technology and Engineering Systems Journal, vol. 6, no. 4, pp. 401–405, 2021. doi: 10.25046/aj060445
- Khaoula Naouaoui, Toufik Cherradi, "Mechanical Characterization of Recycled Aggregates Concrete Based on its Compressive Strength", Advances in Science, Technology and Engineering Systems Journal, vol. 6, no. 3, pp. 35–39, 2021. doi: 10.25046/aj060305
- Sebastianus Bara Primananda, Sani Muhamad Isa, "Forecasting Gold Price in Rupiah using Multivariate Analysis with LSTM and GRU Neural Networks", Advances in Science, Technology and Engineering Systems Journal, vol. 6, no. 2, pp. 245–253, 2021. doi: 10.25046/aj060227
- Hajar Bouazza, Aarti Bansal, Mohsine Bouya, Azeddine Wahbi, Antonio Lazaro, Abdelkader Hadjoudja, "Modeling and Design of a Compact Metal Mountable Dual-band UHF RFID Tag Antenna with Open Bent Stub Feed for Transport and Logistics Fields", Advances in Science, Technology and Engineering Systems Journal, vol. 6, no. 1, pp. 1065–1071, 2021. doi: 10.25046/aj0601118
- Khaoula Naouaoui, Azzeddine Bouyahyaoui, Toufik Cherradi, "Durability of Recycled Aggregate Concrete", Advances in Science, Technology and Engineering Systems Journal, vol. 6, no. 1, pp. 735–741, 2021. doi: 10.25046/aj060180
- Antonio Vitale, Federico Corraro, Nicola Genito, Luca Garbarino, Leopoldo Verde, "An Innovative Angle of Attack Virtual Sensor for Physical-Analytical Redundant Measurement System Applicable to Commercial Aircraft", Advances in Science, Technology and Engineering Systems Journal, vol. 6, no. 1, pp. 698–709, 2021. doi: 10.25046/aj060176
- Imane Jebli, Fatima-Zahra Belouadha, Mohammed Issam Kabbaj, Amine Tilioua, "Deep Learning based Models for Solar Energy Prediction", Advances in Science, Technology and Engineering Systems Journal, vol. 6, no. 1, pp. 349–355, 2021. doi: 10.25046/aj060140
- Thinh Dang Cong, Toi Le Thanh, Hao Mai Tri, Phuc Ton That Bao, Trang Hoang, "Applications of TCAD Simulation Software for Fabrication and study of Process Variation Effects on Threshold Voltage in 180nm Floating-Gate Device", Advances in Science, Technology and Engineering Systems Journal, vol. 6, no. 1, pp. 146–152, 2021. doi: 10.25046/aj060116
- Devika K N, Ramesh Bhakthavatchalu, "Modified Blockchain based Hardware Paradigm for Data Provenance in Academia", Advances in Science, Technology and Engineering Systems Journal, vol. 6, no. 1, pp. 66–77, 2021. doi: 10.25046/aj060108
- Qazi Mudassar Ilyas, Muhammad Mehboob Yasin, "An Anonymity Preserving Framework for Associating Personally Identifying Information with a Digital Wallet", Advances in Science, Technology and Engineering Systems Journal, vol. 6, no. 1, pp. 36–42, 2021. doi: 10.25046/aj060104
- Mahadev Sarkar, Gaurav Anand, Sivakumar Ramadoss, "Wideband Active Switch for Electronic Warfare System Applications", Advances in Science, Technology and Engineering Systems Journal, vol. 5, no. 6, pp. 1312–1321, 2020. doi: 10.25046/aj0506156
- Zdenko Balaž, Krystian Wawrzynek, "Cognitive Cybernetics in the Foresight of Globalitarianism", Advances in Science, Technology and Engineering Systems Journal, vol. 5, no. 6, pp. 718–723, 2020. doi: 10.25046/aj050686
- Mounir Amraoui, Rachid Latif, Abdelhafid El Ouardi, Abdelouahed Tajer, "Feature Extractors Evaluation Based V-SLAM for Autonomous Vehicles", Advances in Science, Technology and Engineering Systems Journal, vol. 5, no. 5, pp. 1137–1146, 2020. doi: 10.25046/aj0505138
- Fatma Mallouli, Aya Hellal, Fatimah Abdulraheem Alzahrani, Abdulsalam Ali Almadani, Nahla Sharief Saeed, "Comparative Study of Cryptocurrency Algorithms: Coronavirus Towards Bitcoin’s Expansion", Advances in Science, Technology and Engineering Systems Journal, vol. 5, no. 5, pp. 452–459, 2020. doi: 10.25046/aj050556
- Vikas Anand Vatul, Arputha Aravinth, Narayanan K, Gulshan Sharma, Tomonobu Senjyu, "A Novel Demand Side Management by Minimizing Cost Deviation", Advances in Science, Technology and Engineering Systems Journal, vol. 5, no. 5, pp. 262–268, 2020. doi: 10.25046/aj050532
- Duc Manh Nguyen, Sunghwan Kim, "A Novel Quantum No-Key Protocol for Many Bits Transfer with Error Correction Codes", Advances in Science, Technology and Engineering Systems Journal, vol. 5, no. 2, pp. 781–785, 2020. doi: 10.25046/aj050298
- Segundo Moisés Toapanta Toapanta, Daniela Monserrate Moreira Gamboa, Luis Enrique Mafla Gallegos, "Analysis of the Blockchain for Adoption in Electronic Commerce Management in Ecuador", Advances in Science, Technology and Engineering Systems Journal, vol. 5, no. 2, pp. 762–768, 2020. doi: 10.25046/aj050295
- Amine Kardi, Rachid Zagrouba, "Attacks classification and security mechanisms in Wireless Sensor Networks", Advances in Science, Technology and Engineering Systems Journal, vol. 4, no. 6, pp. 229–243, 2019. doi: 10.25046/aj040630
- Shreyas K K, Abhishek H P, Sneha M, Shobha G, Deepak Kumar, "Integration of Third-Party Routing Stack to NetScaler CPX", Advances in Science, Technology and Engineering Systems Journal, vol. 4, no. 6, pp. 192–195, 2019. doi: 10.25046/aj040624
- Hamid Ali Abed AL-Asadi, Abdulhadi Alhassani, Nor Azura Ahmed Hambali, Mustafa Abdulazeez AlSibahee, Saif Ali Alwazzan, Ali Mohammed Jasim, "Priority Incorporated Zone Based Distributed Clustering Algorithm for Heterogeneous Wireless Sensor Network", Advances in Science, Technology and Engineering Systems Journal, vol. 4, no. 5, pp. 306–313, 2019. doi: 10.25046/aj040539
- Mohamad Faiz Ahmad, Syed Sahal Nazli Alhady, Ooi Zhu Oon, Wan Amir Fuad Wajdi Othman, Aeizaal Azman Abdul Wahab, Ahmad Afiq Muhammad Zahir, "Embedded Artificial Neural Network FPGA Controlled Cart", Advances in Science, Technology and Engineering Systems Journal, vol. 4, no. 4, pp. 509–516, 2019. doi: 10.25046/aj040461
- Borislava Spasova, Daisuke Kawamoto, Yoshiyasu Takefuji, "Evaluation of the Effects of Bidding Strategy with Customized Pricing on the Individual Prosumer in a Local Energy Market", Advances in Science, Technology and Engineering Systems Journal, vol. 4, no. 4, pp. 366–379, 2019. doi: 10.25046/aj040445
- Segundo Moisés Toapanta Toapanta, Gabriel Enrique Valenzuela Ramos, Félix Gustavo Mendoza Quimi, Luis Enrique Mafla Gallegos, "Prototype of a Security Architecture for a System of Electronic Voting for the Ecuador", Advances in Science, Technology and Engineering Systems Journal, vol. 4, no. 4, pp. 292–299, 2019. doi: 10.25046/aj040437
- Dorathy Abonyi, "A Novel Strategy For Prompt Small Cell Deployment In Heterogeneous Networks", Advances in Science, Technology and Engineering Systems Journal, vol. 4, no. 4, pp. 265–270, 2019. doi: 10.25046/aj040433
- Salwa Salman Abed, Zahra Mahmood Mohamed Hasan, "Multi-Step Iteration Algorithm of Total Asymptotically Quasi-Nonexpansive Maps", Advances in Science, Technology and Engineering Systems Journal, vol. 4, no. 3, pp. 69–74, 2019. doi: 10.25046/aj040311
- Segundo Moisés Toapanta Toapanta, Andrés Javier Bravo Jácome, Maximo Giovanny Tandazo Espinoza, Luis Enrique Mafla Gallegos, "An Immutable Algorithm Approach to Improve the Information Security of a Process for a Public Organization of Ecuador", Advances in Science, Technology and Engineering Systems Journal, vol. 4, no. 3, pp. 25–30, 2019. doi: 10.25046/aj040304
- Syam Madhusoodhanan, Abbas Sabbar, Sattar Al-Kabi, Stanley Atcitty, Robert Kaplar, Binzhong Dong, Jiangbo Wang, Shui-Qing Yu, Zhong Chen, "High-Temperature Optical Characterization of Wide Band Gap Light Emitting Diodes and Photodiodes for Future Power Module Application", Advances in Science, Technology and Engineering Systems Journal, vol. 4, no. 2, pp. 17–22, 2019. doi: 10.25046/aj040203
- Xiaojian Xu, Wanyi Huang, "Optimal Designs of Constrained Accelerated Life Testing Experiments for Proportional Hazards Models", Advances in Science, Technology and Engineering Systems Journal, vol. 4, no. 1, pp. 101–113, 2019. doi: 10.25046/aj040111
- Babacar Dieng, Moussa Toure, Modou Beye, Diouma Kobor, Amadou Seidou Maiga, "Morphological and Optoelectrical Characterization of Silicon Nanostructures for Photovoltaic Applications", Advances in Science, Technology and Engineering Systems Journal, vol. 4, no. 1, pp. 45–49, 2019. doi: 10.25046/aj040106
- Duarte Lopes de Oliveira, Orlando Verducci, Vitor Leandro Vieira Torres, Robson Moreno, Lester de Abreu Faria, "Synthesis of QDI Combinational Circuits using Null Convention Logic Based on Basic Gates", Advances in Science, Technology and Engineering Systems Journal, vol. 3, no. 4, pp. 308–317, 2018. doi: 10.25046/aj030431
- Tao Wu, Ruomei Wang, "Stream Cipher by Reed-Solomon Codes", Advances in Science, Technology and Engineering Systems Journal, vol. 3, no. 4, pp. 26–32, 2018. doi: 10.25046/aj030404
- Peter Arato, Gyorgy Racz, "A Method for Generating, Evaluating and Comparing Various System-level Synthesis Results in Designing Multiprocessor Architectures", Advances in Science, Technology and Engineering Systems Journal, vol. 3, no. 3, pp. 129–141, 2018. doi: 10.25046/aj030318
- Sara Mubeen, Hafiz Muhammad Mudassar Aslam, Muhammad Tamour Danish, Muhammad Imran, Aamira Hashmi, Muhammad Hashim Raza, "Effect of Risperidone with Ondansetron to Control the Negative and Depressive Symptoms in Schizophrenia", Advances in Science, Technology and Engineering Systems Journal, vol. 3, no. 3, pp. 100–103, 2018. doi: 10.25046/aj030313
- Veit Bayer, Stephanie Kunath, Roland Niemeier, Jurgen Horwege, "Signal-Based Metamodels for Predictive Reliability Analysis and Virtual Testing", Advances in Science, Technology and Engineering Systems Journal, vol. 3, no. 1, pp. 342–347, 2018. doi: 10.25046/aj030141
- Solym Mawaki Manou-Abi, William Dimbour, "S-asymptotically w-periodic solutions in the p-th mean for a Stochastic Evolution Equation driven by Q-Brownian motion", Advances in Science, Technology and Engineering Systems Journal, vol. 2, no. 5, pp. 124–133, 2017. doi: 10.25046/aj020519
- Nacer E. El Kadri E, Abdelhakim Chillali, "Simulation of flows in heterogeneous porous media of variable saturation", Advances in Science, Technology and Engineering Systems Journal, vol. 2, no. 5, pp. 70–77, 2017. doi: 10.25046/aj020512
- Zdenko Balaž, Davor Predavec, "Cognitive Cybernetics vs. Captology", Advances in Science, Technology and Engineering Systems Journal, vol. 2, no. 6, pp. 107–118, 2017. doi: 10.25046/aj020614
- Frank Müller, Bernd Bertsche, "Planning an Availability Demonstration Test with Consideration of Confidence Level", Advances in Science, Technology and Engineering Systems Journal, vol. 2, no. 3, pp. 1565–1576, 2017. doi: 10.25046/aj0203195
- Safarul Ilham, Abdul Muin, Dedi Candro, "System Control Device Electronics Smart Home Using Neural Networks", Advances in Science, Technology and Engineering Systems Journal, vol. 2, no. 5, pp. 35–37, 2017. doi: 10.25046/aj020507
- Pryo Utomo, Nadia Widari Nasution, Arisman, Rahmat Widia Sembiring, "A Theoretical and Experimental Comparison of One Time Pad Cryptography using Key and Plaintext Insertion and Transposition (KPIT) and Key Coloumnar Transposition (KCT) Method", Advances in Science, Technology and Engineering Systems Journal, vol. 2, no. 5, pp. 31–34, 2017. doi: 10.25046/aj020506
- Elwinus H. A. Mendrofa, Elwin Yunith Purba, Boy Yako Siahaan, Rahmad W Sembiring, "Collaborative Encryption Algorithm Between Vigenere Cipher, Rotation of Matrix (ROM), and One Time Pad (OTP) Algoritma", Advances in Science, Technology and Engineering Systems Journal, vol. 2, no. 5, pp. 13–21, 2017. doi: 10.25046/aj020503
- Amin Subandi, Rini Meiyanti, Cut Lika Mestika Sandy, Rahmat Widia Sembiring, "Three-Pass Protocol Implementation in Vigenere Cipher Classic Cryptography Algorithm with Keystream Generator Modification", Advances in Science, Technology and Engineering Systems Journal, vol. 2, no. 5, pp. 1–5, 2017. doi: 10.25046/aj020501
- Zubayr Khalid, Pritam Paul, Khabbab Zakaria, Himadri Nath Saha, "An Encryption Key for Secure Authentication: The Dynamic Solution", Advances in Science, Technology and Engineering Systems Journal, vol. 2, no. 3, pp. 540–544, 2017. doi: 10.25046/aj020369
- Fernando Yepes-Calderon, Olivier Beuf, "Homemade array of surface coils implementation for small animal magnetic resonance imaging", Advances in Science, Technology and Engineering Systems Journal, vol. 2, no. 3, pp. 532–539, 2017. doi: 10.25046/aj020368
- Mbanusi Echefuna Cyril, Ngwu Chukwuemeka, Onyeka Festus, Onoh Felix, "Forced Vibration Response of Double-Bay Multi-Storey Building Frames with Joints of Infinite Rigidity", Advances in Science, Technology and Engineering Systems Journal, vol. 2, no. 4, pp. 68–77, 2017. doi: 10.25046/aj020410
- Abdelhakim Chillali, "Matrix Encryption Scheme", Advances in Science, Technology and Engineering Systems Journal, vol. 2, no. 4, pp. 56–58, 2017. doi: 10.25046/aj020408
- Estefanía D. Avalos-Rivera, Alberto de J. Pastrana-Palma, "Classifying region of interests from mammograms with breast cancer into BIRADS using Artificial Neural Networks", Advances in Science, Technology and Engineering Systems Journal, vol. 2, no. 3, pp. 233–240, 2017. doi: 10.25046/aj020332
