Fabrication of Glaze Material from Recycled Bottle Glass and Kaolin
Volume 4, Issue 6, Page No 313-320, 2019
Author’s Name: Agus Dwi Anggonoa), Elkana Bilak Lopo, Joko Sedyono, Tri Widodo Besar Riyadi
View Affiliations
Department of Mechanical Engineering, Universitas Muhammadiyah Surakarta, Jl.Ahmad Yani, PO.BOX 1 Pabelan, Surakarta 57162, Indonesia
a)Author to whom correspondence should be addressed. E-mail: ada126@ums.ac.id
Adv. Sci. Technol. Eng. Syst. J. 4(6), 313-320 (2019); ![]() DOI: 10.25046/aj040640
DOI: 10.25046/aj040640
Keywords: Glaze, Recycle Glass Bottle, Kaolin, Vickers Hardness
Export Citations
This research aimed at developing a method of glaze material making from used glass bottle waste combined with kaolin. Then, the microstructure and hardness level of glaze products were characterized. Furnace heaters were used in research to heat glaze material by varying the holding time of 30, 45 and 60 minutes. The composition of glaze material was varied with the ratio between glass and kaolin of 70:30, 80:20 and 90:10. The results revealed that the process of glaze material making was successfully carried out. The results of the hardness test showed that at the 30 minutes holding time, the hardness was 82.53 VHN. The hardness value increased when the holding time was 45 minutes with an average hardness value of 85.03 VHN. The highest average hardness obtained when the variation of 60 minutes holding time was 87.6 VHN. The difference in hardness value in variation possibly occurred because of the differences in the distribution of amorphous solids, porosity, and pinhole which was influenced by the differences in glass and kaolin composition.
Received: 24 October 2019, Accepted: 22 November 2019, Published Online: 12 December 2019
1. Introduction
Ceramic glass products are widely known for their unusual combination properties which have exotic combinations of material properties that produce a variety of high-tech products according to the market needs [1]. S.D Stookey introduced the term “pyroceram” for ceramic glass (glass code 9606) in which pyroceram is an original ceramic glass material developed and licensed by Corning Glass in the 1957s. Prior to the development of pyroceram, Stookey worked in controlled crystallization in glasses that were sensitive to radiation (glass code 8603) and was named photosensitive opal glass. But in 1959, Stookey adopted and used the term “ceramic glass” for two types of material, not depend on the type of nucleation agent used (Cu, Ag, Au or TiO2) [2].
The term ceramic glass indicates a material that contains a significant crystal volume fraction of > 50%. The volume fraction of ceramic glass crystals is significantly lower than that which has been developed over the past 60 years. Therefore, problems that limit the number of crystalline phases or the composition of ceramic glass have been removed. Aluminasilikat system is widely used as an object of research and has been commercialized as ceramic glass materials. The importance of new features in the development of ceramic glass production is the need for evolution control in various types of crystals simultaneously or independently grow in large numbers or on the surface of the sample [3][4].
Ceramic products have been widely applied in engineering, especially in machineries such as cutting tools, nozzles, valves, turbines, and ball bearing [5]. The advantages of ceramics, in general, are a high melting point, high-temperature resistance, friction resistance, corrosion resistance, low heat conductivity, relatively low density and low thermal expansion coefficient [6]. Currently, ceramic materials have been developed into modern products with very varied advantages. Ceramic base material in the market is usually made from clay which has passed the process of molding, heating, and finishing. Clay is one of the basic ingredients of ceramic which is plastic, easy to print, stiff after being dried and glassy after being heated at a certain temperature. The use of clay as a base for ceramics is largely determined by the nature of the mineral of the clay compilers. The nature of clay minerals is determined by several factors such as chemical composition and mineral types, physical and chemical properties, and the structure of clay minerals so that clay quality test as a ceramic raw material can not only be determined by chemical composition testing, but also must be tested through the test for the types, mineral structures and physical properties. The clay is most commonly used as ceramic materials such as red brick, tile, earthenware, and others. Generally, the tile is made of clay, but it has some weaknesses such as cracking, breaking and selling price is too expensive. So, it is necessary to increase mechanical and physical properties through experimental studies of the material coating.
The data from the Ministry of Environment and the Ministry of Public Works of the Republic of Indonesia in 2017 showed that the total waste in Indonesia reaches 187.2 million tons/ year, where the composition of glass waste reached 17% of 187.2 million tons. Waste is the material that is thrown, will be thrown or no longer used according to its purpose in which a material considered waste if no longer used or it is essentially a kind of waste such as liquid waste. From the data, it can be seen that if the waste cannot be reduced, it will harm the environment and nature. Quoted from ristekdikti.go.id about “how to manage urban waste” written by Agus Puji Prasetyono, it is said that the priority of the city or capital to waste becomes “Apiori” in which apriori is existing knowledge before having experience. The term is used to explain that someone can think and have assumptions about everything before having experience and finally draw conclusions. By this, talk about rubbish means to talk about “how”. Therefore this becomes a guideline or motivation in selecting the research topic. It should be noted that glass waste takes 10-1000 years for the glass to completely decompose. Based on this, concrete steps must be taken to reduce glass waste so that the amount of glass waste does not increase. Several steps to reduce glass waste are implementing the 3R method involving Reuse, Reduce and Recycle. This method has been carried out by several industries, non-governmental organizations, and individuals who care about the environment and want to help to reduce the impact of glass waste on the environment. From those three steps, the quite effective step in reducing the impact of glass waste is recycle stage. Recycle method is the process of turning a used material into a new material to prevent waste. By applying the recycling process, it is expected that glass waste can be utilized as reusable materials and can reduce waste at a certain limit, can save natural resources and reduce dependence on certain raw materials.
The research reported by Celik [7] on ceramics making using mineralogy method and chemical analysis method which is a sample of raw soil was carried out using X-ray diffraction (XRD) techniques to obtain clearer soil patterns. Clay minerals can be expanded and then heated to 550oC for 3 hours to differentiate chlorite and kaolinite. The XRD pattern is obtained from Rigaku Rint-2200 diffractometer that operates at a tube voltage and current of 40kV and 30mA. Related to “Thermal analysis (DTA-TGA)”, Netzsch STA 409 differential calorimeter tool under atmospheric air is used to analyze termal termo gravimetri termal (DTA-TGA). The temperature is raised from room temperature to 1200° C at a speed of 10° C per minute and maintained at this maximum temperature for 10 minutes. In “Dilatometry test”, raw clay is heated to 1200° C at a heating rate of 50o C per minute to 1100o C, and 30oC per minute for temperatures between 1100o C and 1200° C is maintained at a maximum temperature of 10 minutes. Samples that are dried overnight at 105o C were then heated in a Misura dilatometer of 3.32 (ODHT-HSM) in which 1600/80 changes are recorded every minute during heating. From the research, it is concluded that clay from Afyon Instambul is characterized using chemical, mineral and thermal analysis. Afyon clay has the qualities needed to make ceramic tiles and is an alternative ceramic raw material for developing Turkish ceramic tiles.
In [8], the authors reveal that alumina-oriented ceramics with fraction textures ranging from 9.6% to 93.6% are prepared by templated grain growth (TGG) of nanoscale matrices, the fraction texture can be controlled directly on ceramic density, and absorption of grain texture can be observed at ≥ 58.4%. The research used the X-ray diffraction (XRD) method, morphological features can be observed by scanning electron microscopy (SEM), to measure the density using the Archimedes method, the characteristics of mechanical properties are measured by three bends in universal testing, while hardness testing uses Vickers indenter (HVS-5). By this, it is concluded that the microstructure, mechanical properties, and the development period of alumina ceramic textures occur the compaction in the sample. Anisometric granules attachment occurs when orientation of ≥ 58.4%, cracked deflection between surfaces originating from oppressed grains because energy fractures is lower than the surface, in which further enhancements result in longer cracking deflection distances, texture characteristics of 93.6% alumina ceramics substantially increase Kc = 4.6 MPa.m1/2 and σf = 589 MPa, and therefore the luck occurs when the crack deflection.
In [9], the researchers report that the implementation of innovative materials for energy-saving focusing on the building sector is a very interesting topic throughout the world. In this case, aerogel is indicated as a promising material because it has increased in recent years in which aerogel has very low thermal conductivity around 0.01-0.02 W/(m.k). In his paper, he explained that the development of a dual glass panel system is with monolithic silica aerogel. It is mentioned that the results of the multi-disciplinary analysis include thermal and lighting tests. From the results, it is concluded that research carried out in different climates, the aerogel window can be used as a solution to reduce energy demand and even the demand in winter by reducing the increase in solar heat in buildings. It is important to state that when the internal burden on school buildings and office buildings is high, climate adjustment tends to be considered. And moreover, the use of aerogel glass on the window allows a lot of light during the day enters the room which affects on activity and prosperity. The window glass in this study is applied in real life, especially at the risk of condensation and heat changes.
In [10], the authors pinpointed that the characteristics of glaze sanitary physical properties are controlled and improved by changing the chemical composition of raw materials. Glaze industry from ten raw materials has different compositions, by spraying traditional ceramic substrates, then thermally treated and processed at 1250oC. The glaze characteristics obtained are diffracted with X-rays to reveal the mineralogical composition and are confirmed by the FTIR spectrum and observed through images to see the electron distribution to study the microstructure and then, it is found that micro characteristics are obtained in white from the colorimeter data. From the results, it is concluded that the 10% difference in calculations and experiments is derived from the coefficient of thermal expansion and white color increase reaches 87.5%, the maximum zircon content is 14.5wt%, the lowest ZnO content is 2.5wt% and the flexural strength is 47.61 MPa.
The same research has also been reported by Martinez et. al [11] about the development of glaze material with glass and calcite-based material (CaCO3). In this research, variations in holding time are carried out including 15 minutes, 30 minutes and 45 minutes with a combustion temperature of 1000oC using a furnace machine and also the hardness test (Vickers) is carried out. Then, it is concluded that the micro photo showed that at 15 minutes holding time, there is cracking on the ceramic glaze layer, 30 minutes holding time has also cracking but there is a pinhole in the glaze layer, while at the 45 minutes holding time, the pinhole and cracking decreases. In the hardness test, the 15 minutes holding time is 452,761 VHN, the 30 minutes holding time is 479,914 VHN, the 45 minutes holding time is 391,695 VHN. This is done successfully and it obtained the highest results at a holding time of 30 minutes.
According to Demidenko [12], the characterization of calcium silicate ceramics using scanning electron microscopy (SEM) techniques sintered at temperatures of 850oC to 1100oC reveals that the microstructure of calcium silicate ceramics is going better at sintering temperatures of 1100oC with a density of 2.32 g/cm3, and porosity of 27.5%.
In [13], the authors examine the zircon corrosion behavior of glazed ceramic glass against strong acid solutions. It showed that the composition of the glass phase is determined by its glaze resistance. When ZrO2 is involved in the glassy phase, very poor resistance is obtained through the formation of soluble Zr-containing compounds. Enhancing SiO2 content is enhanced the resistance of the glass phase and dissolution of the observed grain or grain boundary phases. The coalescing of ZrO2 into the glass phase must be avoided to increase the chemical resistance of zircon containing glaze. When ZrO2 is in the grains, good chemical resistance can be obtained.
Additionally, In [14], the authors concluded that the mixture chosen from waste and low-cost mineral initial materials can be converted into glass ceramic components either by vitrification and subsequent crystallization or by direct sintering. The sintered-crystallization approach leads to strong glass ceramics, under the simple conditions (pressing fine powder and sintering at 950oC for only 30 minutes). The porosity of the glass-ceramic body surface from direct sintering can be sealed by ceramic glaze which resulted from the same initial mixture of waste and minerals. The characteristics of the waste ceramic glaze can be adjusted by the addition of secondary components (glass panels from disassembled CRT and zircons). Because of their specific strength characteristics, coated ceramic glass can be applied in the building industry as lightweight tiles.
In [15], the authors reported that the milling method used is a technological scheme for the synthesis of ceramic materials using waste material from oil refineries containing heavy metals. This study experimented with the SEM test and revealed that pigments calcined in experiments using temperature of 700, 900, has a morphology that is very agglomerated, while, at a temperature of 1100, agglomerates that have formed undergo structural decomposition. For compositions containing almost all metal iron (92%) turned to magnesium chromium at temperatures of 1000. It is concluded that the proposed alternative technology scheme could provide economical production of ceramic pigments with exceptional compositional stability at relatively low temperatures from calcination.
In [16], the authors reported that glass powders are used as an alternative material to replace cement. In this study, glass powders are as an additive to cement because it depends on the chemical elements present in glass and also in cement, namely silica (SiO2). In this study, it is compared using four mixed compositions, among others 0%, 10%, 20%, 30% with a total of seven specimens each with a 28-day treatment period based on SNI 03-0349-1989 standards. The results indicated that the use of glass powder on bricks with a mixture composition of 10%, 20%, 30% has met the requirements for the water absorption of solid concrete brick quality level I according to the provisions of SNI 03-0349-1989, bricks with a composition of 0% are included in quality level III according to the provisions standard of SNI 03-0349-1989 with an average compressive strength of 58.16 kg/cm2, while bricks with a composition of 10%, 20%, 30% are included in quality level II with an average compressive strength of 74,41 kg/cm2. So, it can be concluded that the most optimum mixture is bricks with a mixture composition of 20%.
In [17], the authors explained that the heat treatment process is an effort to increase the strength and hardness of steel by heating the steel to achieve austenite temperature that is followed by quench so that the martensite phase arises. In principle, the surface treatment of the material is almost the same but only done on the surface of the material in which the main goal is to get a hard surface of the component but the inside of the component remains resilient. From that condition, the method used is induction heating. Principally, it uses heating obtained from Eddy currents caused by flux magnetic originating from the coil of alternating electric current. Furthermore, it is tested using induction heaters for the heat treatment process on the surface of ST 37 steel specimens with the room temperature controlled by thermodigital, while, the specimens are quenched using a water cooling medium.
The manufacture of quality hardflek products from plastic waste and rice husks. The method of processing plastic waste and rice husk into hardflek products as a substitute for wood raw materials in furniture products has a high creative value in which quality and productivity is much better. Furthermore, the economic value of a furniture product is largely determined by the design or shape and appearance offered by the raw material. Plastic waste material combined with rice husk waste provides a very attractive aesthetic nature with interesting color because the coloring has been integrated in the material so that it cannot fade or be degraded by time. Processing methods of products made from polyethilene terepthalate (PET) plastic waste is reinforced with rice husk waste. The method applied can use the system hand lay-up (pouring). The test results showed that the addition of rice husk ash at a volume fraction of 93% plastic waste and 7% rice husk ash has a lower impact strength value than that of the initial specimen (without rice husk) from 0.0024 joules/mm2 to 0.0015 joules/mm2. The hardness testing of the Vickers method showed that the addition of rice husk ash in a volume fraction of 93% plastic waste and 7% of rice husk ash has increased from the initial specimen which is about 35% (10.6 HV to 13.5 HV), so there is a need for research development with SEM (scanning electron microscopy) method to know the composition and properties of the material contained in the product [18].
All things considered, it was necessary to conduct research that utilizes used bottle waste to be used as a more useful material in the form of glaze material. The purpose of this research was to develop glaze material by utilizing glass waste from used bottles combined with kaolin. To find out the characteristics and structure of glaze material, it was conducted the micro photo testing and hardness level analyzing of the glaze material using the Vickers hardness test.
2. Research Methodology
2.1. Materials
This study used bottle waste as the main material to coat the surface of the tile and also kaolin as an additional material in the coating process in which the combustion process used a furnace machine. Three types of time variations used were 30 minutes, 45 minutes and 60 minutes, besides, variations comparison of material composition between glass and kaolin were also used by the comparisons of 90:10, 80:20 and 70:30. This experiment began with the preparation of tile material since the tile material will be coated, followed by cutting the tile with a length of 2 cm, width 2 cm, and thickness 0.8 cm. This process used tile material that had not been burned. Figure 1 showed the tile material that had been cut and sanded using sandpaper ranging from 800, 1000, 1500, 2000 to 5000 grits.
 Figure 1: Clay tile specimens: (a) before being cut, (b) after being cut and mashed
Figure 1: Clay tile specimens: (a) before being cut, (b) after being cut and mashed
Glass pounding machine was used to refine glass from used bottles into powder with a size of 200 meshing. To refine the used bottle glass used a glass pounding machine in an existing glass pulverizer at the Metallurgy Laboratory. Then it was meshed using size 200 mesh. Figure 2 shows the glass powder that had not been meshed and after meshing.
Kaolin (clay) is a clay mineral containing several layers of aluminum silicate. Kaolin is a kind of clay that is smooth, soft and white resulted from weathering granite rocks which is then used as material to make porcelain or to make a mixture of woven fabric (paper, rubber, and medicine, etc.), commonly called Chinese clay. Furnace machine is an equipment used to heat materials and change their shape (eg rolling, forging) or changing its properties (heat treatment). It is commonly referred to as an oven. Energy transfer in the furnace occurred during the heat energy by generation steps through element heater whose energy was supplied from electrical energy. In this study, a furnace machine was used to heat glass powder on a tile.
 Figure 2: Recycle bottle glass (a), Glass powder before meshing (b) and after meshing 200 mesh (c).
Figure 2: Recycle bottle glass (a), Glass powder before meshing (b) and after meshing 200 mesh (c).
The coating process is done manually with a thickness of 1mm. It is done by giving the liquid mixture of glass powder, kaolin, and water on the tile. This to mix glass and kaolin powder so that it can stick on the surface of the tile. The drying process is carried out so that the coating adheres firmly to the specimen. This process is done at room temperature and allowed to stand for a day. The combustion process uses ceramic tile specimens that have been coated with glass powder and kaolin. This process is carried out using a furnace engine involving necessary steps which are when the furnace engine is turned on, set the temperature on the screen with 1000°C, wait for the furnace machine to show the temperature of 1000°C on the screen in front. When the temperature already reaches 1000°C, set the time with a stopwatch for 30, 45, and 60 minutes. When the stopwatch shows the desired time, turn off the furnace machine and leave the specimen in the furnace machine until the temperature drops using room temperature. After the furnace machine reaches room temperature, then take a specimen from the furnace machine, then leave it for a day to proceed to testing.
2.2. Experiments
In the research, to investigate the influence of kaolin contents and holding time to the glaze hardness the micro hardness test was conducted. The experiment is carried out based on the ASTM E384 standard. The force was 0.5 kgf and 10 s of holding time.
Micro-photograph analysis testing was conducted to determine the microstructure of the results of the coating glass process on the tile. The observation is done by obtaining an image that shows the distribution of ceramic material with a zoom of 200 times, and also by arranging the lighting. From this picture, it finds out the properties and characteristics of ceramic materials.
3. Results and Discussion
3.1. Photo Micro
To facilitate reading comprehension in this test analysis, the glaze coating carried out using a variety of composition compared to glass powder and kaolin consisted of (A) 90% : 10%, (B) 80% : 20%, and (C) 70% : 30. There was also a variation of the time ratio performed including 30 minutes, 45 minutes, and 60 minutes, with the same temperature of 1000oC. This was to find out which one is better for use after the rain process.
Figure 3 shows the results of micro-photographs on the surface of the glaze material. Figure 3 is the results of micro-photographs with the variations in the composition of the glaze material with a comparison between glass and kaolin.
 Figure 3: The results of micro-photographs on the glaze material holding time 30 minutes
Figure 3: The results of micro-photographs on the glaze material holding time 30 minutes
From the observation results of micro-photographs tests conducted in Figure 3 (A), number 1 is shown in the form of longitudinal lines like fibers and marked in white. It is the melted glass material and has formed on the glaze layer on the surface of the tile or commonly called amorphous solids. While the black one shown in number 2 is porosity. It can possibly occur when kaolin as a binding material glaze is burning to ash and trapped on the surface of the glaze layer. Then, number 3 shows that the existence of pinholes that are possible to occur due to the release of oxygen during combustion and inside the material contains flammable materials at low temperatures. The oxygen arises in the combustion process because the room in the furnace is not in a vacuum condition, so the materials are combusted first and form holes on the surface of the layer. The same process is also shown in the figure B and figure C in which number 1 shown as the shape of a long line that looks like a fiber and marked white is a glass material that has melted but is somewhat less than what is shown in figure A. It is possible occurs because it affects the material composition, whereas porosity that occurs in figure B and C looks more possible occurs because it has more kaolin. The pinholes shown in figure B and C shows that those are in glaze layer and have a smaller size with a greater amount than in figure A. Figure 3 analysis shows that the use of temperatures of 1000oC during the combustion process successfully melt the glass.
 Figure 4: The results of micro-photographs on the glaze material holding time 45 minutes.
Figure 4: The results of micro-photographs on the glaze material holding time 45 minutes.
Figure 4 shows the results of micro-photographs on the surface of the glaze material. Figure 4 is the result of micro photographs with composition variation of the glaze material with a comparison between glass and kaolin. The longitudinal lines like fibers that are marked in white are melted glass materials or commonly called amorphous solids. It forms a glaze layer on the tile surface, but it can be seen that the melting rate of glass material is not evenly distributed well. Equally, it happens in figure B and C. It can be seen that the melting rate of glass material that occurs in figure B shows that the melting rate of glass material looks more evenly distributed if compared to the figure A and C. This is possible due to differences in the composition of glass and kaolin. Figure A number 2 shows porosity in which it occurs in smaller and fewer sizes. Porosity also occurs in figure B and C, but in figure C the porosity looks less if compared to figure A and figure B. Pinholes that occur in the observation can be seen in number 3, but in part B, it looks smaller pinhole. This possibly happens because it depends on the length of time to hold the combustion and composition comparison.
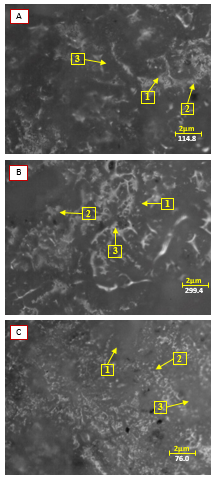 Figure 5: The result of micro-photographs on the glaze material holding time 60 minutes
Figure 5: The result of micro-photographs on the glaze material holding time 60 minutes
Figure 5 shows the results of micro-photographs on the surface of the glaze material. Figure 5 is the result of micro-photographs with variations in the composition of the glaze material with a comparison between glass and kaolin. The observation results of micro-photographs tests conducted in figure A, number 1 is shown in the long lines like fibers and marked in white. It is the melted glass material and has formed on the glaze layer on the surface of the tile or commonly called amorphous solids. But it can be seen that the melting rate of glass material is not evenly distributed properly. Identically, it happens in figure B and C. But it can be seen that the melting rate of glass material that occurs in figure C shows that the melting rate of glass material looks more evenly if compared to figure A and B. This is possible because of differences in the glass and kaolin composition. The observations can be strengthened by other studies [19][2]. Number 2 shown in figure A, B and C shows the porosity, but the porosity seemed less is found in figure C compared to figure A and B. This possibly happens because it affects the composition variation [2]. Pinholes are shown in number 3 of figure A, B and C. In figure A, there is no pinhole compared to figure B and C. This may occur due to differences in composition in figure A and the length of combustion duration.
From the analysis results of micro-photographs on glaze material with a holding time of 30 minutes, 45 minutes and 60 minutes, as well as the variations of material composition comparison between kaolin and glass powder with a ratio of 90:10, 80:20, 70:30, figure 5 part C that has a variation of 70:30 and a holding time of 60 minutes shows the results of a dense glaze layer compared to other variations. It is because the glaze materials have different compositions and holding times.
3.2. Micro Hardness
Figure 6 is the result of the photos of glass material that has been coated and has been through the heating process with the furnace. Then, it will conduct the process of specimen testing of finished material using a hardness test tool.
 Figure 6: Location of the indertor point on micro Vickers hardness testing
Figure 6: Location of the indertor point on micro Vickers hardness testing
Specimens used in this test were tile materials that had been coated with glass powder and kaolin with variation composition after being heated at 1000o C. The combustion aims at increasing the hardness on coated material and the surface a thin layer was formed. After completing the combustion process, a hardness test (Vickers) is conducted to find out the hardness value on the surface of each material. The rectangular diamond pyramid indenter is used in the hardness test with 0.5 kgf and a time of 10 seconds is set as the loading test. The results of the hardness test that have been carried out can be seen in figure 7.
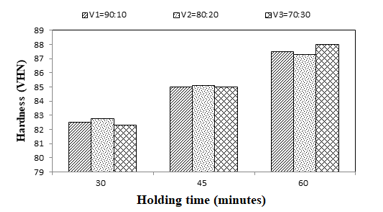 Figure 7: Comparison of glaze hardness values with composition variations
Figure 7: Comparison of glaze hardness values with composition variations
The overall results of the hardness test figure 7 analysis is a variation of the glaze composition between glass and kaolin, and different variations of the holding time. It has been proven by test results in figure 7. It can be stated that the process of a hard layer forming on the surface of tile material that is on the highest Vickers hardness testing occurs at a holding time of 60 minutes and composition variation of 70: 30% with an average hardness value of 88.1 VHN. While the lowest hardness is at a time variation of 30 minutes with an average hardness value of 81.9. The difference in the hardness value in each variation is possible because the characteristics of microstructure formed are also different. This is evidenced in section 4.1 which discusses the results of micro-photographs testing in which the less of the diminishing product defects in the form of pinhole and porosity, the higher the hardness.
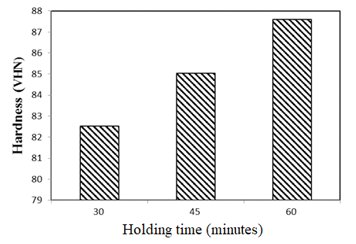 Figure 8: Comparison between the average hardness value (Vickers) with time variations.
Figure 8: Comparison between the average hardness value (Vickers) with time variations.
4. Conclusion
From the results of the analysis and discussion regarding the coating of glaze material using glass and kaolin based materials, it can be concluded that the time variation is very influential on the results of micro-photographs analysis and hardness test. So, the longer the holding time given, the more the melting rate of the glass evenly distributed and the hardness test has also increased. The results of micro-photographs analysis on glaze material samples using glass and kaolin base materials are combusted at a temperature of 1000oC with different variations of holding time of 30 minutes, 45 minutes, and 60 minutes. It can be concluded that the holding time of 30 minutes indicates the glass melt at the holding time of 30 minutes maximum melting at this temperature, and there are still pinholes and porosity in the analysis. At the 45 minutes holding time, it shows that the glass melt has not been evenly distributed since there are some pinholes and porosity but it looks less and smaller than the 30 minutes holding time. At the 60 minutes holding time, the glass melt is still visible but looks smaller and less, porosity and pinhole are still visible but look smaller and less than the holding time of 30 minutes and 45 minutes. The results of hardness test (Vickers) analysis revealed that the hardness value increases in each composition variation carried out. At a holding time of 30 minutes, 45 minutes and 60 minutes, it shows the increase average hardness value of 82.53 VHN, 85.03 VHN, 87.6 VHN.
Conflict of Interest
The authors certify that they have NO affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript.
Acknowledgment
Authors would like to thanks to Universitas Muhammadiyah Surakarta, and Master Program of Mechanical Engineering for supporting the research.
- M. J. Davis and E. D. Zanotto, “Glass-ceramics and realization of the unobtainable: Property combinations that push the envelope,” MRS Bull., vol. 42, no. 3, pp. 195–199, 2017. https://doi.org/10.1557/mrs.2017.27
- J. Deubener, M. Allix, M.J Davis, A. Duran, T.Hoche, T. Komatsu, S. Kruger, I. Mitra, R. Muller, S. Nakane, M.J. Pascual, J.W.P. Schmelzer, E.D. Zanotto, S. Zhou., “Updated definition of glass ceramics,” J. Non. Cryst. Solids, vol. 501, no. January, pp. 3–10, 2018. https://doi.org/10.1016/j.jnoncrysol.2018.01.033
- M. Rampf, M. Dittmer, C. Ritzberger, and W. Höland, “Controlled parallel crystallization of lithium disilicate and diopside using a combination of internal and surface nucleation,” Front. Mater., vol. 3, no. October, pp. 1–9, 2016. https://doi.org/10.3389/fmats.2016.00047
- A. Halliyal, A. Safari, A. S. Bhalla, R. E. Newnham, And L. E. Cross, “Grain-Oriented Glass-Ceramics for Piezoelectric Devices,” J. Am. Ceram. Soc., vol. 67, no. 5, pp. 331–335, 1984. https://doi.org/10.1111/j.1151-2916.1984.tb19532.x
- V. Luntz-Leybman, R. K. Freund, and A. C. Collins, “5A-Pregnan-3A-Ol-20-One Blocks Nicotine-Induced Seizures and Enhances Paired-Pulse Inhibition,” Eur. J. Pharmacol., vol. 185, no. 2–3, pp. 239–242, 1990. https://doi.org/10.1016/0014-2999(90)90648-P
- Yasin Erdoğan, “Physicochemical Properties of Handere Clays and Their Use as a Building Material”, Journal of Chemistry, Volume 2015. http://dx.doi.org/10.1155/2015/374245
- H. Celik, “Technological characterization and industrial application of two Turkish clays for the ceramic industry,” Appl. Clay Sci., vol. 50, no. 2, pp. 245–254, 2010. https://doi.org/10.1016/j.clay.2010.08.005
- M. Zhang, Y. Chang, R. Bermejo, G. Jiang. Y. Sun, J. Wu, B. Yang, W. Cao., “Improved fracture behavior and mechanical properties of alumina textured ceramics,” Mater. Lett., vol. 221, pp. 252–255, 2018. https://doi.org/10.1016/j.matlet.2018.03.123
- U. Berardi, “The development of a monolithic aerogel glazed window for an energy retrofitting project,” Appl. Energy, vol. 154, pp. 603–615, 2015. https://doi.org/10.1016/j.apenergy.2015.05.059
- K. Boudeghdegh, V. Diella, A. Bernasconi, A. Roula, and Y. Amirouche, “Composition effects on the whiteness and physical-mechanical properties of traditional sanitary-ware glaze,” J. Eur. Ceram. Soc., vol. 35, no. 13, pp. 3735–3741, 2015. https://doi.org/10.1016/j.jeurceramsoc.2015.05.003
- Juan Daniel Martínez, Santiago Betancourt-Parra, Ivonne Carvajal-arín, Mariluz Betancur-Vélez, “Ceramic light-weight aggregates production from petrochemical wastes and carbonates (NaHCO3 and CaCO3) as expansion agents,” . Construction and Building Materials, Volume 180, 2018, Pages 124-133, https://doi.org/10.1016/j.conbuildmat.2018.05.281.
- N. I. Demidenko and G. B. Tel’nova, “Microstructure and properties of a material based on natural wollastonite,” Glas. Ceram. (English Transl. Steklo i Keramika), vol. 61, no. 5–6, pp. 183–186, 2004. DOI: 10.1023/B:GLAC.0000043088.65135.11
- G. Topateş, B. Tarhan, and M. Tarhan, “Chemical durability of zircon containing glass-ceramic glazes,” Ceram. Int., vol. 43, no. 15, pp. 12333–12337, 2017. https://doi.org/10.1016/j.ceramint.2017.06.097
- M. A. Binhussain, M. Marangoni, E. Bernardo, and P. Colombo, “Sintered and glazed glass-ceramics from natural and waste raw materials,” Ceram. Int., vol. 40, no. 2, pp. 3543–3551, 2014. https://doi.org/10.1016/j.ceramint.2013.09.074
- M. Doynov, T. Dimitrov, and S. Kozhukharov, “Alternative technological approach for synthesis of ceramic pigments by waste materials recycling,” Bol. la Soc. Esp. Ceram. y Vidr., vol. 55, no. 2, pp. 63–70, 2016. https://doi.org/10.1016/j.bsecv.2016.01.002
- Fernanda Andreola, Luisa Barbieri, Isabella Lancellotti, Cristina Leonelli, Tiziano Manfredini, “Recycling of industrial wastes in ceramic manufacturing: State of art and glass case studies”, Ceramics International, Volume 42, Issue 12, 2016, Pages 13333-13338. https://doi.org/10.1016/j.ceramint.2016.05.205.
- Anggono, A. D., Muttaqiem, Z., Darmawan, A. S., Besar Riyadi, T. W., Yulianto, A., Sugito, B., Abdullah, E. M. (2019). Mechanical Properties of Concrete Block Reinforced with Recycle HDPE and Coal Bottom Ash. Materials Science Forum, 961, 51–56. https://doi.org/10.4028/www.scientific.net/msf.961.51
- A. D. Anggono, B. Sugito, A. Hariyanto and Suranto, “Investigation on mechanical properties of friction stir welding of 2 mm thick aluminium alloy sheet,” 7th Brunei International Conference on Engineering and Technology 2018 (BICET 2018), Bandar Seri Begawan, Brunei, 2018, pp. 1-4. doi: 10.1049/cp.2018.1519
- X. Lu, J. Yang, X. an Ning, K. Shih, and F. Wang, “Crystallization pathways in glass-ceramics by sintering cathode ray tube (CRT) glass with kaolin-based precursors,” J. Eur. Ceram. Soc., vol. 38, no. 15, pp. 5184–5191, 2018. https://doi.org/10.1016/j.jeurceramsoc.2018.06.047
Citations by Dimensions
Citations by PlumX
Google Scholar
Scopus
Crossref Citations
- O.Yu. Fedorenko, N.M. Samoilenko, A.О. Baranova, G.V. Lisachuk, R.V. Kryvobok, "Development of the composition of matte glaze with usage of pharmaceutical glass waste for the production of porcelain stoneware." Voprosy Khimii i Khimicheskoi Tekhnologii, vol. , no. 5, pp. 123, 2023.
- Chaimae Mourou, María Martín-Morales, Montserrat Zamorano, Diego P. Ruiz, "Light Reflectance Characterization of Waste Glass Coating for Tiles." Applied Sciences, vol. 12, no. 3, pp. 1537, 2022.
No. of Downloads Per Month
No. of Downloads Per Country
