Preparation of Lead, Lead Alloy(S) And Other Salts from Exhausted Rechargeable Lead Batteries
Volume 4, Issue 4, Page No 529-538, 2019
Author’s Name: Mahmoud Abdel-Hamed Rabah1,a), Maie Ibrahim Abdul Aziz El-Gammal2, Mahmoud Salem Ibrahim2, Omar Mohamed Helmy Abdul Aziz2
View Affiliations
1Chemical and electrochemical metallurgy, Central metallurgical research and development Institute CMRDI, Helwan,11421, Cairo, Egypt.
2Environmental Science Department, Faculty of Science, Damietta Univ. Egypt
a)Author to whom correspondence should be addressed. E-mail: mrabah010@gmail.com
Adv. Sci. Technol. Eng. Syst. J. 4(4), 529-538 (2019); ![]() DOI: 10.25046/aj040464
DOI: 10.25046/aj040464
Keywords: Rechargeable batteries, Pure lead, Secondary lead-aluminium, Magnesium alloy, Nickel and cobalt, Hydrometallurgy
Export Citations
Metals of lead and some lead alloys, lead oxide, nickel and cobalt were recovered from exhausted battery by combined hydrometallurgy and pyro metallurgical method. The spent batteries were dismantled and leached in hot 2M and 5M nitric acid. The unleached fraction was heated with sodium carbonate to produce lead oxide. Salts in the leached solution were analyzed by Inductively Coupled Plasma ICP. Lead was precipitated as hydroxide on cold with ammonia. Nickel and cobalt metals in solution were extracted by solvent extraction using LEWATIT MP 600 ion exchange resin. Metals loaded by the organic phase were stripped by HCl. Metals hydroxides were reduced with ascorbic acid or hydrazine hydrate to ultrafine free metals, Lead alloys were prepared by encapsulating the alloying metal oxide or organic salts in the host lead metal and heated at 800 °C. The end products were investigated with Energy-dispersive X-ray spectroscopy (EDX), X-ray diffraction (XRD), X-ray fluorescence (XRF), Inductively Coupled Plasma (ICP), and Scanning Electron Microscope (SEM). Results revealed that the spent grids contain 94.2 % lead, aluminium 0.12% nickel 0.05 % and cobalt 0.053 %. The particle size of the reduced metals was found in range of 15-60 um, LEWATIT MP 600 ion exchange resin is specific adsorbent for nickel and cobalt. Distribution constant Kd value of the stripping step decreased in the order Ni and Co. Lead-Al-Mg alloy was prepared by heat treatment of terminal taps at 500 °C. The obtained lead alloys were investigated with EDX and SEM. The extent of recovery of lead metal and lead calcium aluminum alloy amount to 94.3% and 96.4% with high purity. Lead -calcium alloy was homogeneous and contain calcium particles with 5 ums.
Received: 04 March 2019, Accepted: 16 August 2019, Published Online: 25 August 2019
1. Introduction
Rechargeable batteries are usually used for temporary power supply in general and in houses in particular in case the main power shutoff accidentally The Bureau of Mines [1] has investigated an electrolytic recycling process to recover lead and improve secondary recovery of metals and minerals from scrap batteries Metallic fraction of the crushed batteries is directly melted and cast as anodes for electro refining The sludge is leached with ammonium carbonate [(NH4)2CO3] and ammonium bisulfite (NH4HSO3) to convert lead sulfate (PbSO4) and lead dioxide (PbO2) to lead carbonate (PbCO3), . . The lead metal grids and plates are separated from the sludge by ball milling, washing, and screening by the Betts process using waste fluosilicic acid as the electrolyte.
Battery is made of groups of plates connected together by external flag terminal made of lead alloy. Lead oxide(s), sulphate powders filling in the grids openings to form the electrically active material. In the charged state, the negative plate paste is lead-calcium grid loaded with lead sulphate; the positive electrode is lead dioxide. Both of these lead materials are in a spongy form to optimize surface area and thereby maximize the electrical capacity [1] The conductivity media is potassium hydroxide in low quantity just sufficient to moisten the electrode paste. In the discharging state, the negative lead plate loses electrons and got oxidized to a higher lead oxidation state. Lead-calcium alloy provides benefits of good grid density, conductivity, & tensile strength. It reduces water consumption over life of battery, it also reduces electrolyte & hydrogen gas evolution. Better self-discharge characteristics- (typically .05% per day at 25°C) is attained with this alloy together with stable rate under float charge over the life of the battery and constant current draw [2] Chen et al [3] repor4ted recovery of lead from fly ash of waste lead-acid batteries. The lead salt is lead sulphate (PbSO4) and lead oxy sulphate (Pb2OSO4). Nitric acid and sodium hydroxide were used for leaching of the fly ash sample. With S/L of 60 gL-1, the leach extent of Pb was 43% and 67% in 2M acidic and basic solutions, respectively. Anglesite is soluble in NaOH whereas lanarkite is mildly soluble in HNO3. Lead metal was recovered by electrolysis from the leach solution with the help of an electrolytic cell fitted with graphite coated with titanium (Ti-DSA) anodes and stainless-steel cathode. Properties of anodes deposited with lead dioxides were analyzed by cyclic voltammetry… Junqing et al, 2012, [4] recovered method, in which high purity metallic Pb is directly produced by electrolyzing PbO obtained from waste lead acid batteries in alkaline solution A hydrometallurgical process has been proposed [5, 6] to recover valuable metals from spent lithium-ion batteries in citric acid media. A process was reported including the steps of calcinations of a spent paste treated with an alkali carbonate or hydroxide or any mixture thereof, and elemental sulphur at a temperature of up to 600° C., followed by washing with water. The heat treated and washed paste was dissolved in an alkali molten electrolyte, and lead was electro-won from the alkali molten electrolyte. The spent electrolyte was reused in the process [7]. Molten flux salts displayed good thermal stability and solvent properties; these characteristics helped their use in materials preparation [8], solar power plants [9], and the getting rid of paints or coating from metal surfaces [10]. An up to date technology, adapted a molten salt to the smelting processes of antimony and bismuth through the use of sodium hydroxide and a mixed molten salt in the NaOH–Na2CO3 2CaO system [11, 12]. Separation of minor elements using Lewisite exchange resin was reported by Badawy et al. [13]. Yoheeswaran1 et al [14], recovered lead metal from used lead acid batteries, by the hydrometallurgical method. The treatment of used batteries for recovering lead was claimed to be important from the point of view of lead production as well as pollution abatement as otherwise the battery scarp leads to serious disposal problems. The pyrometallurgical and other methods suggested in the past decades, were found to be impracticable, and a new method was investigated. Lead metal recovery from spent lead acid batteries applying an electrochemical method comprising two successive steps; lead leaching and electrode position. It was found that 95% of lead metal was leached by 2M of nitric acid and the electrode position step more than 90% of lead metal could be recovered with low current efficiency from the leaching solution. The method adopted was reported to be promising and had great potential for removal of lead from used lead acid batteries. E-waste generation was growing at about 15% and is expected to cross 800,000 tons per year in 2012 in some countries like India. The composition of spent batteries was very diverse and contained over 1000 different substances, which falls under organic and inorganic fractions [Viraja Bhat, 15]. Yunjian Ma and Kiqyiang Qui [16] investigated a compatible environmental process consisted of hydrometallurgical desulfurization and vacuum thermal reduction to recycle lead. Lead paste was desulfurized with sodium carbonate, by which, the content of sulfur declined from 7.87% to 0.26%. Charcoal was used to reduce the desulphurization lead paste under vacuum. Under the optimized reaction conditions, i.e., vacuum thermal reduction at temperature 850 °C under 20 Pa for 45 min,
The aim of this work is to extract metal lead. Lead aluminium-magnesium alloy and some salts from exhausted plates and grids of electrolyte-free rechargeable lead batteries… Recovery of lead metal was performed using hydrometallurgical and pyro metallurgy whereas the alloying elements of aluminium and Magnesium were extracted by solvent extraction technique, Parameters influencing these processes such as temperature, time, and pH value and mole ratio of the reagents were studied.
2. Materials and Methods
2.1. Experimental Details
A sample of about ten Kg of used rechargeable lead batteries was supplied by the waste collection stores, Cairo. The sample was washed with water and left to dry in normal ambient conditions. Figure 1 shows a photograph of the collected spent rechargeable acid lead batteries
 Figure 1 Different models of the spent batteries used in this study
Figure 1 Different models of the spent batteries used in this study
The chemicals used for leaching, precipitation, separation and salts preparation were chemically pure grade. Nitric, formic, acetic acids and calcium carbonate, oxide and hydroxide. Sodium, and potassium were of ADWIC supplier (Egypt) Table 1 summarizes the properties of chemicals used.
Table 1 Properties of the chemicals used in this study
| Supplier | Purpose | Properties | Product |
|
CH3COOH
Nitric acid
H2SO4
HCl |
90 % SP. Gr. 1.044 – 1.049 SP.Gr.1.18 (AR) Min. assay 36 % Fuming 69 % H2SO4 95-97% Extra pure
SP.Gr.1.18 (AR)
|
Leaching Process
|
Riedel- de Hein ADWIC
Riedel- de Hein
ADWIC |
|
Ca carbonate, EJSF2 Ca oxide |
99.3, 1.6 um 3.34 g/cm3, 1.57 um
|
Synthesis process |
Green Egypt Sigma Aldrich |
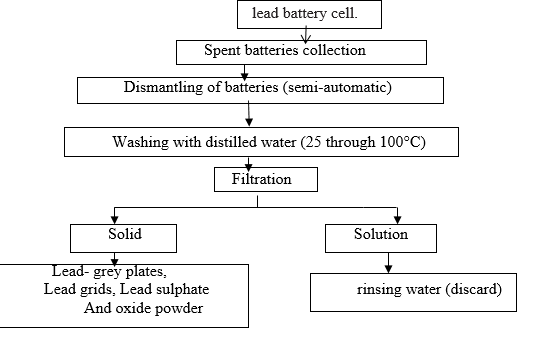 Fig. 2 A conceptual process flow sheet to method to recover lead and lead alloys from exhausted rechargeable acid.
Fig. 2 A conceptual process flow sheet to method to recover lead and lead alloys from exhausted rechargeable acid.
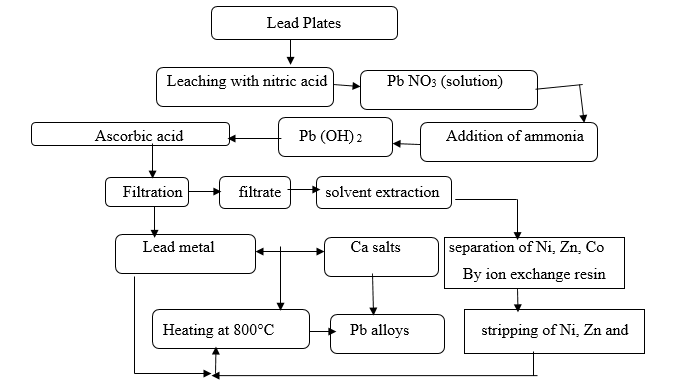 Fig. 3 Process flow sheet for the preparation of lead alloys from spent grids of the battery
Fig. 3 Process flow sheet for the preparation of lead alloys from spent grids of the battery
Organic solvents were used to extract soluble ions of some metals that go into the filtered solution after the leaching process. Table 2 shows the type and properties of the solvents used.
An ion exchange resin Lewatite MP 600 (Merck) was used for ion exchange experiments. It was converted to chloride form before use by thoroughly washing with 0.1-2 M HCl acid in a column for 3 days. It was then washed with aqueous ethanol (70%). The treated resin was then dried under vacuum at 25°C.
A dyestuff of 4-(2-pyridylazo) resorcinol (product of Merck) was used weighing 10 g of molecular weight amounting to 215.21 was used. A 0.02 M stock solution was prepared by dissolving 0.538 g in 0.25-liter 80 % aqueous ethanol. Standard 0.2M bi-sodium hydrogen phosphate Na2HPO4 and 0.1 M citric acid C6H8O7.H2O solutions were used as buffer solutions for pH control.
Table 2 Properties of the solvents used in this work
| Solvent # | Property | Solvent # | Property |
| (1) Acetic acid | (0.1M – 1M) | (3) Acetone /water | 25%-75% |
| (2) Ethanol/ water | 10% – 75% | (4) Diethyl ether | 50 % |
2.2. Method of leaching and preparation of lead-calcium alloy from SLB.
Figures 2 and 3 show a process flow sheet of the applied method to recover lead and lead-alloys from exhausted rechargeable acid.
An ion exchange resin Lewatite MP 600 (Merck) was used for ion exchange experiments. It was converted to chloride form before use by thoroughly washing with 0.1-2 M HCl acid in a column for 3 days. It was then washed with aqueous ethanol (70%). The treated resin was then dried under vacuum at 25°C.
A dyestuff of 4-(2-pyridylazo) resorcinol (product of Merck) was used weighing 10 g of molecular weight amounting to 215.21 was used. A 0.02 M stock solution was prepared by dissolving 0.538 g in 0.25-liter 80 % aqueous ethanol. Standard 0.2M bi-sodium hydrogen phosphate Na2HPO4 and 0.1 M citric acid C6H8O7.H2O solutions were used as buffer solutions for pH control.
2.3. Method of leaching and preparation of lead-calcium alloy from SLB.
Figures 2 and 3 show a process flow sheet of the applied method to recover lead and lead-alloys from exhausted rechargeable acid.
2.4. Determination of lead and other metals in the battery.
Lead. Calcium and other metals content in the exhausted grids were determined by XRF. Leaching was done by 3M nitric acid. The obtained leachate was analyzed by ICP. The unleached residue was also analyzed by XRD.
2.5. Description of the method used to extract minor metals in the leached solution
The method used was given by Badawy et al. [13]. The resin was de-mineralized by packing 1 g of the resin in a glass column through which HCl acid (2M – 0.01 M) was passed for nearly three days to assure that all the resin converts to the chloride form. It was leached with 1 L of 1% (NH4) OH followed by 1 L of 4% sodium sulfate solution. The pH of the prepared metal salt solution(s) (≈ 100 mL) was adjusted to the required value by addition of the buffer solution before starting the adsorption experiments. The pH-adjusted solution was then run through the resin in the column. Ion exchange experiments were conducted by packing 0.2 g Lewisite MP 600 in the column. A solution containing the metal ions was then poured onto the top of the column and allowed to flow at a rate of 0.5 mL/min. The solution was recycled through the column for completely satisfying adsorption of the metal ions.
The effluent was collected in a separating flask. The chloride content in the aliquots from each leach was determined by titration against standard 0.05 N silver nitrate using potassium chromate as an indicator. Stripping of the loaded metals was conducted by eluting with 4 M HCl solution. The collected chloride solutions were separately concentrated by evaporation under vacuum. Chloride was either electrolyzed to prepare the respective metal or converted to insoluble carbonate that was reacted with the acid of concern (inorganic or organic) to prepare the required salt.
Determination of chromium, zinc, cadmium and nickel ions was carried out with the help of a UV-visible atomic absorption spectrophotometer Milton Roy model 20D for the resin and the metals ions determination.
The pH-adjusted solution was then run through the resin in the column. Ion exchange experiments were conducted by packing 0.2 g Lewisite MP 600 in the column. The solution containing the metal ions was then poured onto the top of the column and allowed to flow at a rate of 0.5 mL/min. The solution was recycled through the column for completely satisfying adsorption of the metal ions.
The effluent was collected in a separating flask. The chloride content in the aliquots from each leach was determined by titration against standard 0.05 N silver nitrate using potassium chromate as an indicator. Stripping of the loaded metals was conducted by eluting with 4 M HCl solution. The collected chloride solutions were separately concentrated by evaporation under vacuum. Chloride was either electrolyzed to prepare the respective metal or converted to insoluble carbonate that was reacted with the acid of concern (inorganic or organic) to prepare the required salt.
Determination of chromium, zinc, cadmium and nickel ions was carried out with the help of a UV-visible atomic absorption spectrophotometer Milton Roy model 20D for the resin and the metals ions determination.
The pH value was measured by a based bench pH meter (Hanna model 211) fitted with HFB electrode. Measurements were conducted at 25°C ± 0.2°C.
The exchange capacity of the resin εr was computed from the following relation, Kunin, [17].
![]() Where V is the volume, N is the normality, W is the weight of the resin sample.
Where V is the volume, N is the normality, W is the weight of the resin sample.
The distribution coefficient Kd was determined from the relation given by John et al. [18]
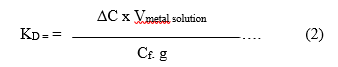 Where ΔC is the change in concentration of the metal in solution before (Ci) and after the experiment (Cf), V is the volume and g are the weight of the resin. Sorption extent (%) was determined from the relation reported by Fethiye and Erol 2005 [19].
Where ΔC is the change in concentration of the metal in solution before (Ci) and after the experiment (Cf), V is the volume and g are the weight of the resin. Sorption extent (%) was determined from the relation reported by Fethiye and Erol 2005 [19].
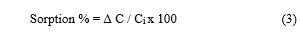 2.6. Method of preparation of the metals and their salts
2.6. Method of preparation of the metals and their salts
The exhausted batteries under investigation were dissolved in 1.1 stoichiometric ratios of 3 M nitric acid at room temperature till complete dissolution (usually takes time). The volume of the solution as adjusted to 250 ml by distilled water. The nitrate solution was treated with an ammonia solution. The prepared nitrate or hydroxide salts were used to prepare free metals of lead or base metals by reduction with ascorbic acid or to prepare carbonate, chloride, sulphate, format, oxalate, citrate and acetate salts…
3. Results and Discussion
Fig. 4 shows the XRD of the lead paste. It is seen that the major content is lead sulphate, lead oxide while lead dioxide is minor. It is seen that major peaks appeared with intensity > 600 at 2θ 20-35 are assigned to the presence of lead sulphate, lead oxide PbO and lead dioxide PbO2. These compounds were also detected with lower intensity (<200) at 2θ ?50 -< 80. Fig 5 shows the XRD pattern examined at low 2θ value of 18 and more. Fig. 5 confirms the presence of minor elements of nickel and cobalt. Table 3 confirms the presence of these minor elements. It is worth noting that lead alloy with this composition is recommended in the manufacture of batteries to help adequate mechanical and thermal stability of the battery during recharging and discharging operations.
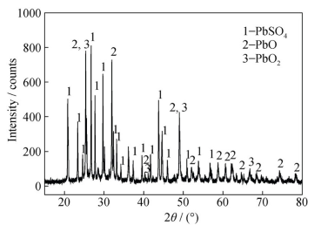 Figure 4 The XRD of the lead paste 46
Figure 4 The XRD of the lead paste 46
It is seen that lead metal conistitutes the major element that amounjts to 94.2 % by weight. Other elements are in minor percentages. It is also seen that the weight percentage of these minor elements decreases in the order Si, Al, Ru, Zr, Co Ni, Cu and Zn. The common elements in commercial use aovered by solvent extraction technique.
This is very clear that lead compounds in grey plates were lead sulphate. The lead compounds in the brown plates were lead oxide and partly lead dioxide. Lead compounds decreased in the order sulphate, oxide and dioxide. Concerning the minor compounds, Ni and cobalt were present nearly in equal amounts (Fig. 3). Table 3 shows the elements present in the exhausted plates as detected by XRF analysis.
It is seen that lead metal is the major element that constitutes 94.2 % by weight. Other elements are in minor percentages. It is also seen that the weight percentage of these minor elements decreases in the order Si, Al, Ru, Zr, Co-Ni, Cu and Zn. The common elements in commercial use are Ni and Co and Zn. These were recovered by solvent extraction.
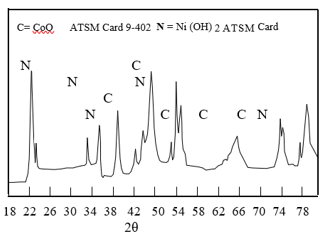 Figure 5 XRD pattern of the minor elements in the battery positive plates
Figure 5 XRD pattern of the minor elements in the battery positive plates
Table 3 The XRF analysis of the elements present in the exhausted plate
| Metal | % wt. |
|
Lead Nickel zinc Cobalt Copper Zirconium Rhobidium Aluminium Silicon
|
94.2 0.05 0.0030 0.053 0.0272 0.0727 0.0842 0.12 0.24 |
technique. Fig. 6 shows the extent of leaching lead from the spent plates using 2M and 5M nitric acid at room temperature. The use of sulphuric acid instead of nitric acid was discarded because the reaction stopped rapidly because lead sulphate is immiscible in water. It can be seen that the leaching process takes time to react with lead compounds. The extent of leaching increases with time to give the maximum extent of 38.4 % after 7 days with 2N acid. More concentrated nitric acid (5M) gives less extent of leaching (32 %). using 2M and 5 M nitric acid at 80 °C shows that 5M nitric is more reactive as compared to 2M concentration. After 6 days the maximum extent of leaching amounts to 38$ and 14 $ with 5M and 2M respectively
Figure 7 shows the effect of time on the leaching extent of the brown paste of the battery using 3M and 5M nitric acid at 80 C. It can be seen that the extent of leaching increases linearly with increase in time attaining a value of about 40% after 6 days. The corresponding value taking place with 3M acid amounts to about 10%. Figure 8 shows the effect of nitric acid concentration on leaching lead sulphate and lead oxide at room temperature for 6 days. It is seen that lead sulphate displays a higher extent of leaching compared to lead oxide up to 5 days. After 6 days lead oxide is readily more leached as compared to lead sulphate.
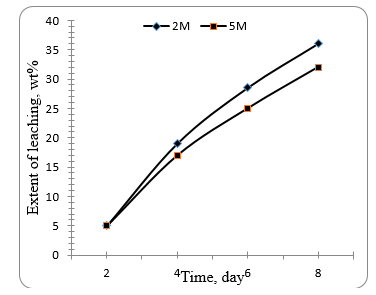 Figure. 6 Effect of time on the leaching extent of grey grids using 2 M and 5 M nitric acid at room temperature
Figure. 6 Effect of time on the leaching extent of grey grids using 2 M and 5 M nitric acid at room temperature
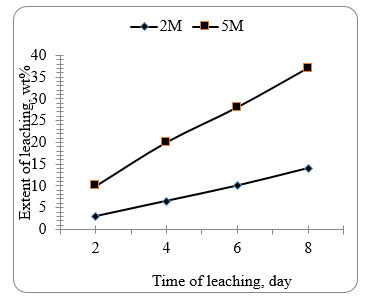 Figure 7. The effect of time on the extent of acid leaching of brown paste using 2M and 5M nitric acid at 80°C
Figure 7. The effect of time on the extent of acid leaching of brown paste using 2M and 5M nitric acid at 80°C
Fig. 9 shows the effect of temperature on the leaching extent of lead oxide present in the battery. It can be seen that increasing the leaching temperature increases the leaching extent. The optimum acid concentration is 3.5 M.
Fig. 10 shows the coefficient of distribution Kd value of Zn ions extracted from the leaching solution using the exchange resin Lewatite. with different concentration of Zn as affected by the amount of the ion exchange resin added at room temperature. It can be seen that the coefficient extent decreases regularly with the corresponding increase in the amount of the used resin and the concentration of Zn ions in the solution.
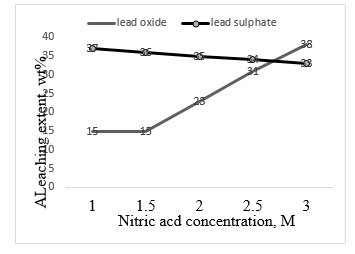 Figure 8 Effect of nitric acid concentration on leaching lead sulphate and lead oxide at room temperature for 6 days.
Figure 8 Effect of nitric acid concentration on leaching lead sulphate and lead oxide at room temperature for 6 days.
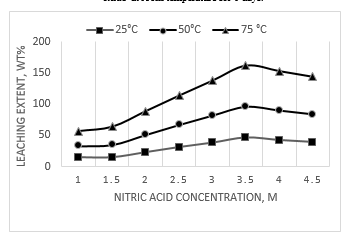 Figure 9 Effect of temperature on the leaching extent of lead oxide present in the battery.
Figure 9 Effect of temperature on the leaching extent of lead oxide present in the battery.
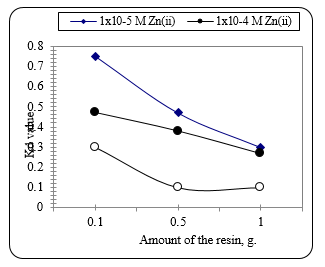 Figure 10 The Kd value for Zn from solution with different concentration of Zn as affected by the amount of the ion exchange resin
Figure 10 The Kd value for Zn from solution with different concentration of Zn as affected by the amount of the ion exchange resin
Fig. 11 shows The Kd value for Co from solution with different concentration of Co as affected by the amount of the ion exchange resin. It can be seen that with and the same metal ion concentration in the solution, the Kd value increases with the decrease in concentration. This statement is logic as given in equation (2). The value of the denominator decreases that gives a corresponding increase in Kd value. The effect of change in g value is less significant as compared to the change in C value.
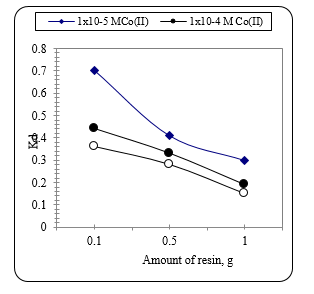 Figure 11 The Kd value for Co extraction from solution with different concentration of Co as affected by the amount of the ion exchange resin
Figure 11 The Kd value for Co extraction from solution with different concentration of Co as affected by the amount of the ion exchange resin
Figure 12 shows the effect of pH value on the Kd value with nickel ions. It can be seen that the effect of pH becomes significant at values ≥ 8.5. At pH 9 the Kd value is highest. Fig. 12 confirms the same findings obtained with Zn ions given in Fig. 13.
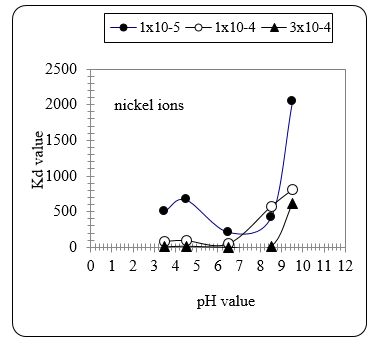 Figure 12 The Kd value for Ni extraction from solution with different concentration as affected by the pH value of the extraction medium
Figure 12 The Kd value for Ni extraction from solution with different concentration as affected by the pH value of the extraction medium
Fig. 14 shows the effect of type of solvent on the obtained metal powder after stripping. It iss seen that solvent 2 gives Ni and Al in nanosize. Solvent 1 gives the same effect with Aluminium. Other solvents give metals with larger size.
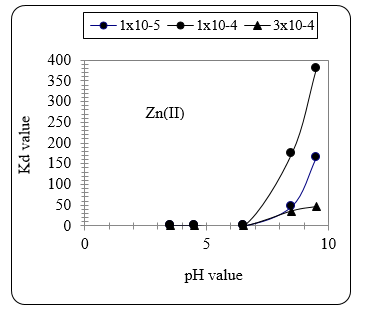 Figure. 13 Effect of pH value on the Kd value with Zn ions.
Figure. 13 Effect of pH value on the Kd value with Zn ions.
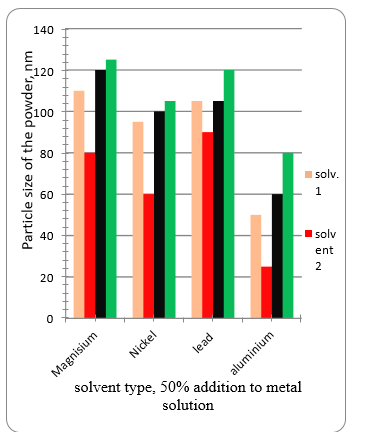 Figure. 14 The effect of type of solvent on the obtained metal powder after stripping
Figure. 14 The effect of type of solvent on the obtained metal powder after stripping
Table 3 shows that lead content in the battery amounts to 94.2 % by weight together with other 8 elements in a minor percentage. Leaching process in this study was carried out at room temperature to save energy although it takes time. The chemical reactions to leach the detected metals taking place according to
Table 4 shows the prepared salts from the spent battery and its purity extent. Table 5 shows the chemical composition of the residue
Table 4 the prepared salts of lead and its purity extent
| Lead salt | Added reagent | Prepared salt |
Purity of product
|
||||
| Lead nitrate | Sulphric acid | Lead sulphate | 99.6 | ||||
| HCl | Lead chloride | 99.2 | |||||
| Sod. carbonate | L:ead carbonate | 100 | |||||
| lead hydroxide | Acetic acid | Lead acetate | pure | ||||
| Oxalic acid | Lead oxalate | pure | |||||
| Formic acid | Lead format | pure | |||||
| Citric acid | Lead citrate | pure | |||||
The unleached fraction remaining after nitric acid leaching was analyzed by XRF.
According to the law of mass action, increasing the nitric acid concentration in the reactants would enhance the leaching extent of lead oxide whereas lead sulphate was unleached. This finding finds support from the fact that sulphate radical is strong to be leached with nitric acid at room temperature. The leaching process is judged by the results given in Fig. 8. The nitrate salt was converted to hydroxide with the action of ammonia solution. This step was necessary to reduce the hydroxide gel to elemental nanoparticles of lead by ascorbic acid as follows:
Reduction of metals hydroxides
Ascorbic acid reduces hydroxide salts of lead and basic metals to free fine particles as follows
Pb (OH)2 + 2 ascorbic acid 2H2O + Pb + 2 oxidized ascorbic acid.
Lead hydroxide + 2 ascorbic acid water lead metal oxidized ascorbic acid
Table 5 the chemical composition of the residue
| Plate | Composition |
|
Grey Brown |
Lead sulphate 100% Lead oxide PbO/PbO2 |
Lead metal in battery plates is present in three forms; a grid made of lead alloy in the form of net-shaped structure loaded with lead sulphate or lead oxide-free metal. Heating the terminal tap of the battery gives lead alloy composed of lead-aluminium-magnesium as revealed from EDX pattern confirmed with SEM image given in Fig. 14. It is composed of 95.83 % wt Pb 1.25% Mg and 2.92 % Al. SEM/EDX is a combination tool, with two instruments working in partnership [20]. These two instruments operate simultaneously to complement each other’s data acquisition, guided by the instrumental operator. Scanning electron microscopy (SEM) provides an image (i.e., morphological information or surface features) on a magnified scale—X 10 to X 100,000, although the usual range is perhaps X50 to X5000. Energy dispersive x-ray (EDX) determines the elemental composition of an area, with a sensitivity of perhaps 0.1 to 1 per cent composition and with a spatial resolution of 1 µm. EDX is commonly used for elements with atomic number ≥11 (sodium), but thin window EDX systems can also detect elements with atomic number ≥5 (boron). With these two instruments operating together, the instrumentalist can scan areas of potential interest, zoom in with higher magnification, and determine elemental compositions in selected areas of interest.
Lead is a soft and ductile metal and lead alloys are used on a large scale. The alloying elements may be antimony, tin, arsenic, and calcium are. Antimony is used to give greater hardness and strength, as in storage battery grids (0.5 to 25%), sheet, pipe, and castings. Antimony contents in the lead-antimony alloys are usually 2 to 5%.
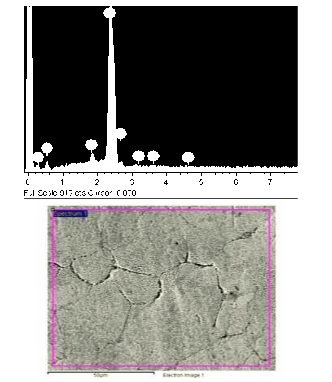 Figure 15 (a) The EDX of the melted terminal tap, (b) the SEM of Pb Al alloy.
Figure 15 (a) The EDX of the melted terminal tap, (b) the SEM of Pb Al alloy.
Fig. 15 shows the EDX and SEM images of lead obtained by melting the terminal tap of the battery under an inert atmosphere. Fig 15 {a} is the EDX pattern of a lead alloy of the terminal tap. Fig. 15(b) is the SEM of the Pb Al. Alloy after annealing at 500 °C Results revealed that only 15-20 % of lead is obtained by simple heating of all the battery plates even in reducing conditions. It becomes legitimate to apply the modified method capable to produce the high extent of lead recovery. In this context, Yoheeswaran1 et al., [15], reported that the recovery of 95% of lead metal from lead-acid batteries by 2M of nitric acid It is found that 95% of lead metal was leached and more than 90% of lead metal can be recovered by electrochemical method with low current efficiency from Pan the leaching solution.
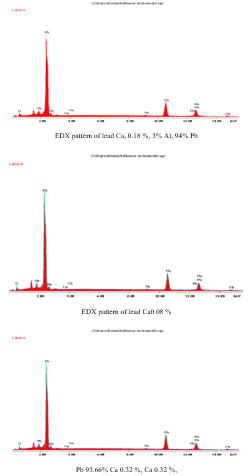 Figure. 16 EDX pattern of lead alloys containing different alloying elements.
Figure. 16 EDX pattern of lead alloys containing different alloying elements.
The method adopted seemed promising and had great potential for removal of lead from used lead-acid batteries. Fig. 16 shows the EDX pattern of lead alloys with calcium (0.018% and aluminium 0.03%, lead alloyed with 0.08% calcium and 0.32% calcium. In this pattern, the main electron beam appeared at 3.00 Kev is stable at a relative height of 66.2 % with all alloys is assigned to elemental lead. The spectra of the alloying elements appeared at 11 Kev with lower relative height pf 10 % and 7 % are for Al and Calcium respectively [22].
Unified Numbering System (UNS) designations for various pure lead grades and lead-base alloys is as follows [23].
- Pure leads L5xx00 – L5xx99
- Lead – calcium alloys L5x700 – L5x899
- Lead – strontium alloys L552xx – L55299
Lead-calcium alloys are used in several applications, particularly, storage battery grids and casting applications. Pb-Ca alloys contain 0.03 to 0.15% Ca. aluminum is now added to calcium-lead and calcium-tin-lead alloys to stabilize calcium. Alloying tin to lead increases hardness and strength. However, lead-tin alloys are usually used for their good melting, casting, and physical properties as in type metals and solders.
4. Conclusion
The output conclusion of this work is that exhausted rechargeable batteries are essential resource for recovery of nonferrous precious metals like lead and lead alloys. Other metals value (Ca. Al, Ni, Co) are present and were successfully recovered. The method used to achieve the goal of this study was simple and friendly environmentally. Preparation of lead metal was matched by a combined hydrometallurgy and pyrometallurgy. The battery was dismantled and the plates were separately leached in nitric acid whereby the nitrate salt was converted to hydroxide by ammonia solution. The hydroxide was reduced to metal lead nanoparticles by ascorbic acid, Alternatively, the lead sulphate and oxide packing the grids were thermally heated with carbon at about 900 ۥ°C to give free metal lead. Alloys of lead with calcium and aluminium has been matched applying the combined hydrometallurgy and pyro metallurgy methods. The extent of recovery amounts to 94.3 % with pure lead and 99.6% with lead alloys of aluminium and calcium. Valuable salts of lead including organic salts were also prepared in highly pure grade.
- E. R, Cole A. Y. Lee. D.L. Paulson,“Recovery of Lead from Battery Sludge by Electrowinning”, JOM, August 1983, Volume 35, Issue 8, pp 42–46 , 1983
- A., Smaniotto, A. Antunes, I. doNascimento, L. Filho D. Luciana, D. Venquiaruto. D. Oliveira, M. , AltemirMossi H. Treichel and R. Dallago J. hazmat..07. pp 26. 2009.
- C. S Chen, Y.J2, Shih, Y.H. Huang “Recovery of lead from smelting fly ash of waste lead-acid battery by leaching and electrowinning. m Waste Management, 52:212-20, 2016
- Junqing Pan, ChaoZhang Yanzhi Sun Zihao Wang “A new process of lead recovery from waste lead-acid batteries by electrolysis of alkaline lead oxide solution”, Electrochemistry Communications. V 19, June 2012, pp 70-72, 2012.
- X., Chen, “Hydrometallurgical process for the recovery of metal values from spent lithium-ion batteries in citric acid media”, Waste management and research J. Vol 32, Issue 11, 2014. https://doi.org/10.1177/0734242X14557380
- D. Andrews, A, Raychaudhuri, and C. Frias. Environmentally sound technologies for recycling secondary lead, J. Power Source, 88. V1, p. 124., 2000.
- X. Chen, , Hydrometallurgical process for the recovery of metal values from spent lithium-ion batteries in citric acid media, WASTE MANAGEMENT AND RESEARCH J. Vol 32, Issue 11, 2014
- E. Margulis, (Hafia, IL), Process for the recovery of lead from spent batteries, USA Patent 4058396, 10/27/ 1998
- G.Z. Chen, D.J. Fray, and T.W. Farthing, Direct electrochemical reduction of titanium dioxide to titanium in molten calcium chloride, Nature, 407(6). 2000
- D. Zhou, C.Y. Zhao, and Y. Tian, Review on thermal energy storage with phase change materials (PCMs) in building applications, Appl. Energy, 92 p. 593. 2012
- Y.Y. Long, J.Z. Li, D.H. Timmer, R.E. Jones, and M.A. Gonzalez, Modeling and optimization of the molten salt cleaning process, J. Clean. Prod., 68, p. 243. 2013
- J.G. Yang, C.B. Tang, Y.M. Chen, and M.T. Tang, Separation of antimony from a stibnite concentrate through a low-temperature smelting process to eliminate SO emission, Metall. Mater. Trans. B, 42 No. 1, p. 30.2011
- N. Badawy, M. A. Rabah and R. Hasan 2013 Recovery of some heavy metals from electroplating rinsing wastewater by LEWATIT MP 600 ion exchange resin., Int. Journal of Environment and waste management, IJEWM, 12(1) 33-51. DOI: 10.1504/IJEWM. 054778. 2013
- Y.J.-Chu, and G. T. Townsend, “Leaching of Lead from Computer Printed Wire Boards and Cathode Ray Tubes by Municipal Solid Waste Landfill Leachates Department of Environmental Engineering Sciences, University of Florida, Gainesville, Florida 32611-6450 Environ. Sci. Technol., 2003, 37 (20), pp 4778–478 2003
- E. Yoheeswaran1, S. Govindaradjane, and T. Sundararajan, “Recovery of Lead Metal from Lead Acid Battery by Hydrometallurgical Method”, International Journal of Engineering Science and Innovative Technology (IJESIT) Volume 2, Issue 1, January 2013
- Viraja Bhat et., al, “Development of an integrated model to recover precious metals from electronic scrap – A novel strategy for e-waste management, Procedia social and behavioral 37, 397-406, 2012.
- Y. Ma and K, Qui “Recovery of lead from lead paste in spent lead acid battery by hydrometallurgical desulfurization and vacuum thermal reduction”, Waste Management 40. 03, 010,2 015
- R. Kunin, A., Rohn. and H. Company (Eds), Philadelphia Pennsylvania, New York, John Wiley and Sons, Second Edition, 1170.2016
- E,G, John, W., Gary and A., Georgiann Preconcentration of trace metal ions by combined complexation-anion exchange Part I. Cobalt, zinc and cadmium with 2-(3′-sulfobenzoyl)-pyridine-2-pyridylhydrazone. Anal. Chim. Acta , 81, 349. 1976.
- G. Fethiye and P. Erol Removal of Cr (VI) from aqueous solution by two Lewatit-anion exchange resins. Journal of Hazardous Materials. 119, (1-3), 175-182. 2005.
- E.R., Bagherian, F. Ongchang, M., Cooper, et al., “Effect of antimony addition relative to microstructure and mechanical properties of continuous cast lead alloy”, Metal, May 25th – 27th 2016, Brno, Czech Republic. 2016.
- M. T. Riaz, Y. Fan, J. Ahmad, M. A. Khan, and E. M. Ahmed, “Research on the Protection of Hybrid HVDC System,” in 2018 International Conference on Power Generation Systems and Renewable Energy Technologies (PGSRET), 2018, pp. 1–6.
- https://en.wikipedia.org/wiki/Unified numbering system.
