Investigating The Detection of Intention Signal During Different Exercise Protocols in Robot-Assisted Hand Movement of Stroke Patients and Healthy Subjects Using EEG-BCI System
Volume 4, Issue 4, Page No 300-307, 2019
Author’s Name: Maryam Butt1,a), Golshah Naghdy1, Fazel Naghdy1, Geoffrey Murray2, Haiping Du1
View Affiliations
1School of Electrical Computer and Telecommunications Engineering, University of Wollongong (UOW), 2522, Australia
2School of Medicine, University of Wollongong (UOW), 2522, Australia
a)Author to whom correspondence should be addressed. E-mail: mb077@uowmail.edu.au
Adv. Sci. Technol. Eng. Syst. J. 4(4), 300-307 (2019); ![]() DOI: 10.25046/aj040438
DOI: 10.25046/aj040438
Keywords: Intention detection, EEG, Brain-computer interfaces, MRCP, AMADEO hand robot, Support vector machines, Stroke
Export Citations
Improving the hand motor skills in post-stroke patients through rehabilitation based on movement intention derived signals from the brain in conjunction with robot-assistive technologies are explored. The experimental work is conducted using Electroencephalogram based Brain-Computer Interface (EEG-BCI) system and the AMADEO hand rehabilitation robotic device. Two protocols using visual-cues and then using a 2-Dimensional (2D) interactive game is presented on a computer screen to healthy subjects as well as post-stroke patients performing the hand movements. The movement intention signals during hand movement are detected through the Support Vector Machine (SVM) classifier. The intent signals produced at six distinct electrodes are investigated to determine electrodes contributing most to the SVM classifier’s performance. Overall, the game protocol shows better classification results for both healthy and stroke patients compared to the visual-cues protocol. FC3 is found to be the most consistent electrode site for the detection of the motor intention of the hand for both protocols. In the experimental work, average classification accuracy for the visual-cues protocol of 67.56% for healthy subjects and 56.24% for stroke patients were obtained. For the game protocol, the classifier accuracy produced for healthy participants was 79.7% and for the post-stroke patients was 66.64%. The results confirm that the intention signal is more pronounced during more engaging activities, such as playing games, for both healthy and stroke subjects. Therefore, the effectiveness of rehabilitation therapy for post-stroke patients could be significantly enhanced using interactive and engaging exercise protocols.
Received: 17 May 2019, Accepted: 20 July 2019, Published Online: 07 August 2019
1. Introduction
This paper is an extension of work presented in 18th IEEE International Conference on Bioinformatics and Bioengineering (BIBE) [1]. Stroke is the main cause of prolonged disability among adults [2]. The most common impairment occurs when a stroke victim has a motor loss of limb(s) on one side of the body causing difficulty with walking and ability to perform activities of daily living. There are many types of rehabilitation therapies currently being practiced, and others being investigated. One of the therapies types being investigated and coming into clinical practice is Robot-Assisted therapy. Advancement in robotic technology over the last decade has led to increasing interest in this type of therapy. For example, Ang et al. [3] used the MIT-Manus robot for a randomized control study with 26 stroke patients to restore their arm movement. Similarly, a haptic knob robot is being used for arm rehabilitation of stroke survivors with positive outcomes [4]. A state-of-the-art robotic device called AMADEO, designed for fine finger motor skill improvement was tested on eight cortical stroke patients and resulted in a 35% increase in their hand movement after multi-session training [5]. All these strategies of post-stroke rehabilitation depend on some neurological adaption that occurs in the patient’s brain to restore the impaired function of the limb. The brain’s ability to adapt to new neural changes, even in adulthood, is called the neuroplasticity or brain plasticity [6].
Brain-Computer Interface (BCI) has an ability to utilize neuroplasticity mechanisms to improve motor function of post-stroke patients during their rehabilitation process [7]. Enhanced motor intention improves motor execution which in turn is fed back to the rehabilitation process. The mechanism of neuroplasticity reinforces the neural pathways which control the movement, leading to functional and motor recovery of the patients [8]. Brain signals that have been used in BCI systems include Electroencephalogram (EEG), Magnetoencephalography (MEG), ElectroCorticoGraphy (ECoG), as well as functional Near-InfraRed Spectroscopy (fNIRS). Amongst these modalities, EEG is the most common modality employed to drive various BCI systems [9]. It is popular because it is not an invasive approach to record the brain’s electrical activities, and because EEG signals have the highest resolution in time-domain [10].
Many studies demonstrate applications of EEG-BCI systems to detect movement intention for various upper-limbs, such as movements of the arm [11-15], elbow [16], [17] and wrist [18] and hand [19-23] for post-stroke rehabilitation.
A self-paced reaching arm movement with the help of a haptic device has been studied in [11] and [12]. In a single-trial EEG protocol, the intention of reaching movement was detected about 500 ms before the actual limb movement [11]. In the follow-up study, the movement directions were decoded up to 76% accuracy for healthy subjects and up to 47% accuracy for stroke patients performed with their impaired arm [12]. Ibanez et al. [13] recruited six healthy subjects and six stroke patients to detect the movement onset of voluntary arm reaching action. The results showed that the True Positive Rate (TPR) of the classifier for healthy participants was 74.5±13.8% while for stroke patients, TPR was 82.2±10.4%. In another study, four chronic post-stroke patients participated in eight training sessions based on arm reaching actions [14]. The intention for the movement was decoded using an EEG-BCI system which then triggered Functional Electrical Stimulation (FES) for further assistance to perform the task. The authors stated that their protocol correctly classified about 66% of movements with an average detection latency of 112±278 ms. Moreover, clinical tests and kinematics results proved the feasibility of the designed intervention for post-stroke recovery.
Among many classifiers, Support Vector Machine (SVM) classifier is commonly used in intent detection of limb movement. For example, Frisoli et al. [15] proposed a gaze-based robot-assisted arm movement protocol in which the real-time movement of a robotic device was controlled by the intended signal of subjects. A Motor Imagery (MI) based EEG-BCI system was used to detect the intention signal through SVM. The authors reported a classification accuracy rate of 89.4±5% for robot-based movement mode. Similarly, Hortal et al. [16] measured the classification performance of SVM by designing a hybrid EEG-BCI system composed of an exoskeleton device and FES for elbow movement. The hybrid BCI system was tested using two training protocols which were MI-based training protocol and a training protocol based on motor intention detection. In the first training protocol, the authors achieved a classification accuracy of about 83% for healthy subjects and 65.3% for stroke patients, while in the second training protocol, the accuracy achieved was approximately 77% and 71.6% for healthy and patient groups, respectively.
Asynchronous EEG-BCI systems are also being deployed for motor intent detection of upper limbs. The triggering of a robotic device through intention signal was studied by Bhagat et al. [17] for elbow joint movement using an asynchronous EEG-BCI robot-assisted system. The motor intent signal of four chronic stroke patients was detected which then triggered an exoskeleton device called MAHI-EXO-II to encourage and guide the active participation of subjects. The intention signal was detected on average −367±328 ms before the actual motor execution. Bai et al. [18] also developed an asynchronous BCI protocol for real-time and online prediction of the self-paced movement of the wrist. Seven healthy subjects were recruited in the study to perform an extension of their wrist whenever they wanted. The authors successfully predicted the voluntary movement at approximately 0.6 s before the real execution of the movement.
The latest applications of EEG-BCI systems have been in detecting intention for movement of the hand, which has a greater complexity of movement than other upper limbs. The hand occupies the largest cortical representation in the motor cortex of the brain [24] as well as playing the most vital role in our daily life activities. Muralidharan et al. [19] distinguished resting state from the extension of fingers by using an open-loop EEG-BCI system. They detected attempted finger extension by post-stroke patients at about 200 to 600 ms before the actual movement onset. Only one EEG channel has been used by Jochumsen et al. [20] to detect motor intent signal during palmar grasp action using handgrip dynamometer. The signal was then decoded into speed and force. They showed that approximately 75% of the movements were detected 100 ms before movement onset, and approximately 60% of task-related movements were accurately decoded into the force and speed levels according to the performed task movements. In a separate study, Jochumsen et al. [21] also distinguish between the intention for three types of grasp tasks (palmar, pinch and lateral) achieving accuracy of 79%, 76%, and 63% respectively during 1-class, 2-class and 3-class classification problems. Ofner et al. [22] classified several arm movements which include hand-opening, hand-closing, forearm supination, forearm pronation, elbow extension as well as elbow flexion. The authors state that one movement can be classified from another with 55% classification accuracy. This research group achieved the classification accuracy of 93% while classifying grasping actions of hand from its rest state compared to the classification results stated by Jochumsen et al. in [21] [23].
In this study, we combine detection of motor intention signals using EEG-BCI system with robot assistive technologies to improve the effectiveness of the hand motor skills in post-stroke patients. Our first stage of research based on an EEG-BCI system and the AMADEO finger-hand rehabilitation robotic device (Tyromotion GmbH, Graz, Austria) is reported. The experimental setup is designed for classification of movement intention of hand-closing versus rest state. It consists of two distinct protocols, which we refer to as protocol A and protocol B. Protocol A, termed as visual-cues protocol, consists of hand-opening and hand-closing pictures which are presented to subjects. In protocol B subjects played an AMADEO game called Shoot-out. We utilize SVM, which is the most common supervised learning algorithm used for these classification problems. Intention signal produced at different single electrodes’ positions during hand movement is analyzed to determine the electrode that is most consistent for the accuracy of SVM classifier.
The rest of the paper is organized as follows. In section 2, the experimental platform and experimental set up are introduced. In section 3, the pre-processing of the signals produced in the experimental work and the classifications method, the classification performance metrics are described. In section 4, the results of the validation of the approach on stroke patients and benchmarking against the healthy subjects are presented. Some conclusions are finally drawn in section 5.
2. Experimental Design and Platform
2.1. Participants
The participants in the experimental work consisted of healthy subjects and post-stroke patients. The healthy subjects consist of four male participants with a mean age of 28 years and no history of any neurological disorder. Two post-stroke patients both right hand dominant were initially assessed through two commonly used clinical tests, namely the Motor Assessment Scale (MAS) and Fugl-Meyer Assessment (FMA) scale. Table I shows each patient’s demographic details as well as their clinical tests scores. The Ethics committee at the University of Wollongong approved all methods and procedures performed in this experiment (Ethics application number: 2014/400). All participants provided written informed consent before this study commenced.
2.2. AMADEO Finger-Hand Rehabilitation Device
The AMADEO finger-hand rehabilitation device is an end-effector robot-assistive system specifically developed for hand movement recovery of post-stroke patients as shown in Figure 1 [25]. It has five Degrees-of-Freedom (DoF) allowing both passive and active movements of fingers as well as the thumb. The robot can generate various patterns of fingers and thumb movements as well as subjects can interact with the robotic device through 2D games.
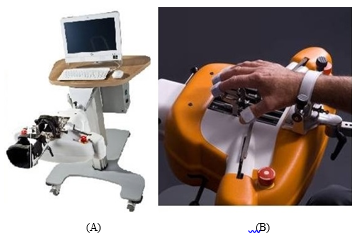 Figure 1: (A) AMADEO Finger-Hand Rehabilitation Unit (B) Hand-Arm Adjustment Support
Figure 1: (A) AMADEO Finger-Hand Rehabilitation Unit (B) Hand-Arm Adjustment Support
2.3. Acquisition of EEG Signal
The EEG signals were acquired by deploying a 32 electrodes Ag/AgCl Quick-Cap (Compumedics-Neuroscan) in accordance to the 10-20 system for positioning electrodes. The electrode positioning diagram of the Quick-Cap is given in Figure 2. The Grael 4K EEG amplifier was employed in this study which has been set to 2048 Hz of sampling frequency. Of the 32 channels, the FPz electrode was used as the ground electrode and the CPz was set as the reference electrode. Thirty remaining electrodes were used for the acquisition of brain signal. In addition, the two electrodes were placed below the left eye and on the supraorbital ridge to record vertical eye movements and eye-blinks. Moreover, two other electrodes placed over the outer canthus of both eyes were used to monitor horizontal movements of eyes.
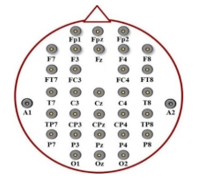 Figure 2: Quick-Cap Electrodes Positions
Figure 2: Quick-Cap Electrodes Positions
2.4. Experimental Setup
Participants were seated upright on a comfortable chair with their right arms attached to the AMADEO hand-arm adjustment support unit. During protocol A, each participant was trained to focus on visual-cues displayed on a computer screen. Visual-cues displaying hand-opening and hand-closing pictures to alert subjects to perform these specific hand movements. The hand-closing pictures were displayed every 5 s, followed by the hand-opening pictures 1 s later with a 4 s waiting period. This resulted in 5 s gap between any two hand-closing visual-cues. Each set comprised 23 trials of hand movements during this protocol.
Of the games available on the AMADEO system, the ‘Shoot-out’ was chosen for use in protocol B. The playing screen of Shoot-out game is shown in Figure 3. In this game, the subject closes their hand to shoot the drum coming out at equal time intervals. Subjects have up to 23 trials of the hand movements in each block.
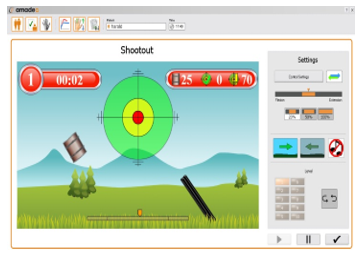 Figure 3: AMADEO Shoot-out Game
Figure 3: AMADEO Shoot-out Game
Table 1: Stroke Patients’ Details and Clinical Test Scores
| Gender | Age | Stroke type |
Time since stroke (months) |
Lesion location | Paretic Hand |
MAS Hand Score (0-6) |
FMA Hand Score (0-14) |
| Female | 64 | Ischemic | 7 | Left pons | Right | 2 | 9 |
| Male | 60 | Ischemic | 4 | Left pons & left frontal regions | Right | 2 | 8 |
All participants performed 6 blocks consisting of 23 trials (6 x 23 = 138) for both protocols. At each hand-closing event, manual event markers were sent to the CURRY 8 (Compumedics, Neuroscan) software which was used for EEG acquisition. Protocol B was the Shoot-out game of AMADEO, which was more interesting and interactive compared to protocol A. We explored the hypothesis in this study that whether a stronger motor intention signal can be produced and detected through SVM during protocol B than protocol A.
3. Signal Processing for Motor Intention Detection
The intention signal during hand-closing movements was detected by offline analysis of the data for both healthy and stroke subjects. The movement intention of a limb produces special EEG patterns in specific parts of the brain and such slow event-related potential is called Movement Related Cortical Potential (MRCP). It appears in the delta frequency band of EEG signal as a direct-current shifts up to 2 s prior to cue-based as well as self-initiated movements [26]. When the person performs the required motor action, the MRCP disappears. The continuous EEG signal at the selected electrodes is divided into two types of epochs. The first, containing MRCP signal is called Move epoch and the second, which does not have an MRCP signal, is named as No-Move epoch. Different time-domain features of MRCP signal are extracted from both these epochs. SVM algorithm is then employed to classify Move and No-Move epochs and, therefore, detecting intention signal of the hand movement.
3.1. Pre-processing
The first step in pre-processing is to filter the EEG signal for the frequency range between 0.1-1 Hz [17] as this narrow range of delta band best captures the anticipatory based MRCP [27]. This band-pass filtering was applied in three steps: first, a high-pass filter with cut-off frequency (fc) of 0.1 Hz was applied; second, for re-referencing of signals, a Common Average Reference (CAR) filter was applied to all signals; and third, a low-pass filter with fc of 1 Hz was applied. Both low-pass and high-pass filters were 4th order Butterworth causal filters. After filtering, the signals were down-sampled from 2048 Hz to 20 Hz to increase computational efficiency [17]. The CURRY 8 (Compumedics-Neuroscan) software was used to perform all the aforementioned pre-processing steps.
3.2. Epoch Extraction & Channel Selection
For further processing, the data were imported into a MATLAB toolbox known as ‘EEGLAB’. Generally, MRCP signal does not exist 2 s before the onset of movement, all Move epochs were extracted from continuous EEG data between -2 s to 0 s with 0 s indicating the instance of actual motor execution. Between 0.5 s to 2.5 s, No-Move epochs were extracted when subjects opened or relaxed their hands. Therefore, epoch length was fixed at 2 s for both epochs. All Move epochs were inspected visually for artifacts, for instances eye-blinks, head movement, and other movement-related artifacts, and then these were removed from the data.
The EEG electrodes located over the left hemisphere of the brain were only chosen because the right-hand movement was considered in this experiment. The electrodes selected were C3, FC3, CP3, Cz, T7 and a Short Laplacian (SL) channel calculated using the C3-(FC3+Cz+CP3+T7)/4 formula [28]. In literature, the C3 channel is most commonly used for right-hand motor intention detection using the MRCP signal. The four neighboring electrodes of the C3 channel and their linear combination were also investigated to determine the best electrode choice for each protocol. It is possible that the negative peak of the MRCP exists before -1.5 s is due to artifact presence. Therefore, every Move epoch was again visually inspected and such Move epochs and their counter-part No-Move epochs were deleted to get the final processed data which was then used to extract features of MRCP signal.
3.3. Feature Extraction & Classification
The two time-domain features from both the Move and No-Move epochs were extracted. These features were the negative peak and the slope of the MRCP signal. The Move epochs have prominent negative peak and slope of MRCP signal compared to No-Move epochs. Based on these time-domain features, the SVM classifier differentiates between both epochs. These were plotted and outliers were removed (along with their counter-class features) before applying the SVM. This prevented biasing the results of SVM due to outliers. In each protocol, an average of 10±2 epochs was rejected per subject. The Move class was marked with a label of ‘1’ and the No-Move class was marked with a label of ‘0’. Depending on these input features, the SVM classified between class 1 and 0. Three-fourths of the dataset was utilized for training data while one-fourth was used as test data for classification.
3.4. Evaluation of SVM Classifier Performance
The SVM classifier’s performance was determined based on classification accuracy percentage, True Positive Rate (TPR)-also known as sensitivity and True Negative Rate (TNR)-also called specificity. They were calculated using the relationships given in (1), (2) and (3) where True Positive, False Positive, True Negative and False Negative were abbreviated as TP, FP, TN, and FN respectively.
Accuracy=(TP+TN)/(TP+TN+FP+FN) (1)
TPR=TP/(TP+FN) (2)
TNR=TN/(TN+FP) (3)
Receiver Operating Characteristics (ROC) curve was analyzed to determine the classifier’s performance during protocol A and B. Moreover, Area Under the Curve (AUC) for the ROC curve was calculated for both protocols. In the end, all these performance metrics were compared to find out which protocol helps better to maintain stronger motor intention level in both healthy subjects and stroke patients and therefore, obtaining better classification results. In addition, these results also could show which electrode was the best choice for intent detection during protocol A and protocol B.
4. Results & Discussion
The experimental work demonstrated the utility of the AMADEO device for rehabilitation of post-stroke patients, while also performing the motor intention detection using the described SVM algorithm.
Six electrodes which include C3, FC3, CP3, Cz, T7, and SL were chosen to determine the importance of electrode selection in intent detection during each protocol. Performance metrics were calculated after the SVM application on test data, including classification accuracy, TPR, TNR and AUC for ROC.
4.1. Intent Detection of Stroke Patients
The performance of the SVM algorithm for detection of hand motor intention signal produced by the stroke patients is studied in this sub-section. Table II presents the accuracy of the SVM classifier at all six selected electrodes for protocols A and B. For protocol A, the FC3 electrode shows the maximum accuracy of the SVM classifier of about 72%. Whereas, the same electrode shows classification accuracy of 89% for intent detection when stroke patients perform the hand movement during protocol B. This supports our hypothesis that during protocol B, subjects are more involved in performing hand movement and are likely to have greater classification accuracy as compared to protocol A. The same trend is demonstrated by the results of C3, CP3, T7, and SL electrodes. However, for electrode Cz, protocol B is showing lower classifier’s accuracy than that for protocol A. The list of electrodes based on the classification accuracies from high-to-low for protocol A is FC3, Cz, SL, C3, T7, and CP3. A similar list for the protocol B is FC3, SL, C3, CP3, T7, and Cz. It is clear from the classifier accuracy results that for both protocols, FC3 shows the maximum classifier performance. However, for protocol B the classifier’s accuracy is higher than that for protocol A at all electrode sites except Cz.
4.2. Intent Detection of Healthy Subjects
The results of the classifier accuracy for healthy subjects for protocols A and B are presented in Table III. The analysis of data for healthy subjects shows that protocol B has better accuracy of SVM classifier than protocol A, except for the channels Cz and T7. With respect to the results acquired for FC3, protocol B has an accuracy of SVM classifier of 98% while the classification accuracy of protocol A is 86%. From Table III, it is clear that the classifier’s accuracy varies in accordance with the selected electrode. For protocol A, the electrodes can be listed as FC3, Cz, C3, T7, SL, CP3 electrodes can be ranked in order of their effectiveness in determining the intent signal. Similarly, for protocol B, the ranking is FC3, C3, SL, CP3, Cz, T7.
Table 2: Accuracy of SVM Classifier for Post-Stroke Patients
| Channel | SVM Classifier’s Accuracy (%) | |
| Protocol A | Protocol B | |
| C3 | 52.88 | 81.01 |
| CP3 | 26.25 | 60.16 |
| FC3 | 72.22 | 88.89 |
| Cz | 70.45 | 34.56 |
| T7 | 49.29 | 51.43 |
| SL | 66.33 | 83.78 |
Table 3: Accuracy of SVM Classifier for Healthy Subjects
| Channel | SVM Classifier’s Accuracy (%) | |
| Protocol A | Protocol B | |
| C3 | 64.58 | 92.86 |
| CP3 | 57.7 | 76.3 |
| FC3 | 86.25 | 98.0 |
| Cz | 73.81 | 70.0 |
| T7 | 63 | 57.69 |
| SL | 60.0 | 83.33 |
When the Cz electrode from stroke patients’ data as well as the Cz and T7 electrodes from healthy subjects’ data were chosen, protocol A had slightly better classification accuracy than protocol B. There could be many possible reasons for this apparent contradiction. The artifact removal is performed using visual inspection method so it might be possible that the data at Cz and T7 electrodes still contain some artifacts in the test data used for protocol B that made the classifier accuracy for protocol B less than protocol A. Moreover, the outlier values removal form Cz and T7 electrodes might cause loss of large data points for protocol B and due to fewer test data values, the performance of protocol B has deteriorated.
4.3. Other Performance Metrics of the SVM Classifier
The accuracy alone cannot justify the performance of the classifier, therefore, sensitivity (TPR) and specificity (TNR) parameters should be also considered. The TPR and TNR values show whether the extracted features in Move and No-Move classes are distinct enough to be classified accurately by SVM. Figure 4 shows bar graph representations of TPR and TNR using stroke patients’ data from protocol A at all six electrodes. Similarly, Figure 5 shows TPR and TNR values at all selected electrodes for protocol B. For protocol A, Figure 4 shows that TNR for CP3, FC3, Cz, and SL is significantly lower than its corresponding TPR. On the other hand, in the case of T7, TNR is slightly higher and slightly lower for C3. However, for protocol B, TNR is higher than TPR for each choice of electrode except for CP3 and T7 as shown in Figure 5.
Similarly, TPR and TNR values using data of healthy subjects for both protocols is shown in Figure 6 and Figure 7. In the case of protocol A, Figure 6 demonstrates that TPR obtained for C3, Cz and FC3 is higher than its corresponding TNR, whereas, for CP3, T7, and SL, the reverse is the case. Figure 7 shows that TPR, for all six electrodes, is higher than its corresponding TNR for protocol B indicating that the Move class contains the adequate features to correctly detect motor intention signal.
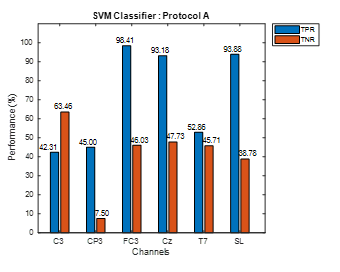 Figure 4: Protocol A Stroke Patients Data
Figure 4: Protocol A Stroke Patients Data
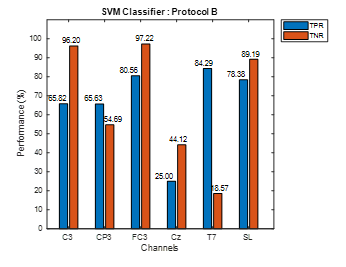 Figure 5: Protocol B Stroke Patients Data
Figure 5: Protocol B Stroke Patients Data
ROC curve is another way of comparing the performance of the SVM classifier for protocols A and B. Figure 8 shows the ROC curve for the FC3 channel, the most appropriate channel for intent detection of hand movement for this experiment, for healthy participants which shows that protocol B has superior performance.
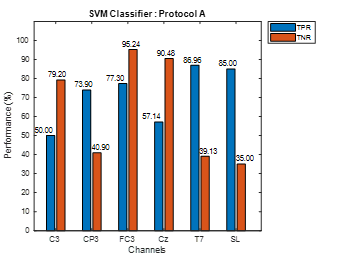 Figure 6: Protocol A Healthy Subject Data
Figure 6: Protocol A Healthy Subject Data
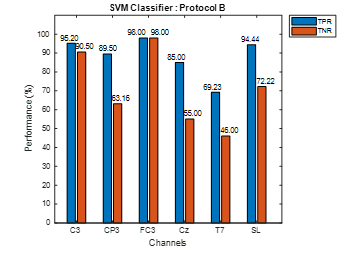 Figure 7: Protocol B Healthy Subject Data
Figure 7: Protocol B Healthy Subject Data
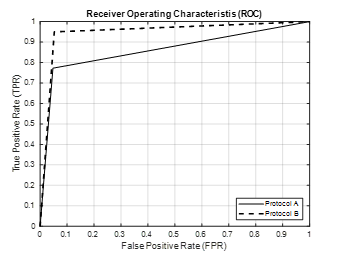 Figure 8: ROC Curve Using FC3 Channel
Figure 8: ROC Curve Using FC3 Channel
The Area Under the Curve (AUC) for ROC is also an important factor in assessing the classifier performance. The excellent classifier has an AUC value of 1 whereas 0.5 value shows its random guessing behavior. The AUC values for FC3 channel for both healthy subjects and stroke patients are listed in Table IV. It is found that AUC for protocol B is greater than protocol A for both healthy subjects and stroke patients which was the expected outcome. Moreover, AUC value for protocol B in the healthy group is ~0.95 which is approximately equal to ideal AUC value for the classifier.
Table 4: Area Under the ROC Curve for FC3 Channel
| Group | AUC for FC3 Channel | |
| Protocol A | Protocol B | |
| Stroke | 0.7222 | 0.8889 |
| Healthy | 0.8626 | 0.9487 |
5. Conclusion
The utilization of EEG-BCI system together with AMADEO robotic rehabilitation system for the functional recovery of the hand of the post-stroke patients was reported as the first stage of our work. The movement intention for both healthy participants as well as post-stroke patients was detected using SVM classifier. Two protocols that were based on simple visual-cues and the AMADEO 2D game were used to detect the hand-closing movement intention vs the resting state. This was followed by an evaluation of the SVM classifier performance by selecting different single electrodes. The average classifier accuracy of 67.56% for the visual-cue protocol and 79.7% for the gaming protocol was achieved for healthy subjects. Similarly, for stroke patients, the classifier accuracy obtained was 56.24% and 66.64% for the visual-cue and the game protocols respectively. The results demonstrated that the 2D games, for example, AMADEO Shoot-out were better activities in retaining concentration compared to static pictures using visual-cues. We conclude that gaming scenarios are preferable for robot-based rehabilitation exercises to promote the active participation of the patients. The FC3 electrode was found to be the best single electrode for the designed experiment with both protocols. Both TPR and TNR were important in determining the performance of the classifier, as these parameters could indicate whether good results of the classifier were obtained either by detecting positive class or negative class accurately. In addition to TPR and TNR, it was shown that the ROC plot and AUC parameters of the classifier could also be used as the performance metric evaluators for the classifier.
Conflict of Interest
There is no conflict of interest among authors.
Acknowledgment
This work is performed by joint support by The University of Wollongong and Higher Education Commission (HEC) Pakistan.
- M. Butt, G. Naghdy, F. Naghdy, G. Murray, and H. Du, “Investigating Electrode Sites for Intention Detection During Robot Based Hand Movement Using EEG-BCI System,” in 2018 IEEE 18th International Conference on Bioinformatics and Bioengineering (BIBE), 2018, pp. 177-180: IEEE.
- J. Adamson, A. Beswick, and S. Ebrahim, “Is stroke the most common cause of disability?,” Journal of stroke and cerebrovascular diseases, vol. 13, no. 4, pp. 171-177, 2004.
- K. K. Ang et al., “A Randomized Controlled Trial of EEG-Based Motor Imagery Brain-Computer Interface Robotic Rehabilitation for Stroke,” Clinical EEG and Neuroscience, Article vol. 46, no. 4, pp. 310-320, 2015.
- K. K. Ang et al., “Brain-computer interface-based robotic end effector system for wrist and hand rehabilitation: Results of a three-armed randomized controlled trial for chronic stroke,” Frontiers in Neuroengineering, Article vol. 7, no. JUL, 2014, Art. no. 30.
- X. Huang, F. Naghdy, H. Du, G. Naghdy, and G. Murray, “Design of adaptive control and virtual reality-based fine hand motion rehabilitation system and its effects in subacute stroke patients,” Computer Methods in Biomechanics and Biomedical Engineering: Imaging & Visualization, vol. 6, no. 6, pp. 678-686, 2018.
- B. B. Johansson, “Brain plasticity and stroke rehabilitation: the Willis lecture,” Stroke, vol. 31, no. 1, pp. 223-230, 2000.
- U. Chaudhary, N. Birbaumer, and A. Ramos-Murguialday, “Brain–computer interfaces for communication and rehabilitation,” Nature Reviews Neurology, vol. 12, no. 9, pp. 513-525, 2016.
- M. Grosse-Wentrup, D. Mattia, and K. Oweiss, “Using brain–computer interfaces to induce neural plasticity and restore function,” Journal of neural engineering, vol. 8, no. 2, p. 025004, 2011.
- H.-J. Hwang, S. Kim, S. Choi, and C.-H. Im, “EEG-based brain-computer interfaces: a thorough literature survey,” International Journal of Human-Computer Interaction, vol. 29, no. 12, pp. 814-826, 2013.
- J. S. Kumar and P. Bhuvaneswari, “Analysis of Electroencephalography (EEG) signals and its categorization–a study,” Procedia engineering, vol. 38, pp. 2525-2536, 2012.
- E. Lew, R. Chavarriaga, S. Silvoni, and R. Millan Jdel, “Detection of self-paced reaching movement intention from EEG signals,” (in eng), Front Neuroeng, vol. 5, p. 13, 2012.
- E. Y. L. Lew, R. Chavarriaga, S. Silvoni, and J. D. Millan, “Single trial prediction of self-paced reaching directions from EEG signals,” (in English), Frontiers in Neuroscience, Article vol. 8, p. 13, Aug 2014, Art. no. 222.
- J. Ibanez et al., “Detection of the onset of upper-limb movements based on the combined analysis of changes in the sensorimotor rhythms and slow cortical potentials,” (in English), Journal of Neural Engineering, Article vol. 11, no. 5, p. 12, Oct 2014, Art. no. 056009.
- J. Ibáñez et al., “Low latency estimation of motor intentions to assist reaching movements along multiple sessions in chronic stroke patients: a feasibility study,” Frontiers in neuroscience, vol. 11, p. 126, 2017.
- A. Frisoli et al., “A new gaze-BCI-driven control of an upper limb exoskeleton for rehabilitation in real-world tasks,” IEEE Transactions on Systems, Man and Cybernetics, Part C (Applications and Reviews), vol. 42, no. 6, pp. 1169-1179, 2012.
- E. Hortal, D. Planelles, F. Resquin, J. M. Climent, J. M. Azorin, and J. L. Pons, “Using a brain-machine interface to control a hybrid upper limb exoskeleton during rehabilitation of patients with neurological conditions,” Journal of Neuroengineering and Rehabilitation, vol. 12, Oct 2015, Art. no. 92.
- N. A. Bhagat et al., “Design and optimization of an EEG-based brain machine interface (BMI) to an upper-limb exoskeleton for stroke survivors,” Frontiers in Neuroscience, Article vol. 10, no. MAR, 2016, Art. no. 122.
- O. Bai et al., “Prediction of human voluntary movement before it occurs,” Clinical Neurophysiology, vol. 122, no. 2, pp. 364-372, 2011.
- A. Muralidharan, J. Chae, and D. Taylor, “Extracting attempted hand movements from EEGs in people with complete hand paralysis following stroke,” Frontiers in neuroscience, vol. 5, p. 39, 2011.
- M. Jochumsen, I. K. Niazi, D. Taylor, D. Farina, and K. Dremstrup, “Detecting and classifying movement-related cortical potentials associated with hand movements in healthy subjects and stroke patients from single-electrode, single-trial EEG,” Journal of neural engineering, vol. 12, no. 5, p. 056013, 2015.
- M. Jochumsen, I. K. Niazi, K. Dremstrup, and E. N. Kamavuako, “Detecting and classifying three different hand movement types through electroencephalography recordings for neurorehabilitation,” Medical & biological engineering & computing, vol. 54, no. 10, pp. 1491-1501, 2016.
- P. Ofner, A. Schwarz, J. Pereira, and G. R. Müller-Putz, “Upper limb movements can be decoded from the time-domain of low-frequency EEG,” PloS one, vol. 12, no. 8, p. e0182578, 2017.
- A. Schwarz, P. Ofner, J. Pereira, A. I. Sburlea, and G. R. Müller-Putz, “Decoding natural reach-and-grasp actions from human EEG,” Journal of neural engineering, vol. 15, no. 1, p. 016005, 2017.
- A. Nakamura et al., “Somatosensory Homunculus as Drawn by MEG,” NeuroImage, vol. 7, no. 4, pp. 377-386, 1998.
- Z. Yue, X. Zhang, and J. Wang, “Hand rehabilitation robotics on post stroke motor recovery,” Behavioural neurology, vol. , 2017.
- H. Shibasaki and M. Hallett, “What is the Bereitschaftspotential?,” Clinical neurophysiology, vol. 117, no. 11, pp. 2341-2356, 2006.
- G. Garipelli, R. Chavarriaga, and J. d. R. Millán, “Single trial recognition of anticipatory slow cortical potentials: the role of spatio-spectral filtering,” in 2011 5th International IEEE/EMBS Conference on Neural Engineering, 2011, pp. 408-411: IEEE.
- D. J. McFarland, L. M. McCane, S. V. David, and J. R. Wolpaw, “Spatial filter selection for EEG-based communication,” Electroencephalography and clinical Neurophysiology, vol. 103, no. 3, pp. 386-394, 1997.
